TriNetX retrospective 1,131 vaccinated COVID-19 patients treated with paxlovid and matched controls, showing lower mortality and hospitalization with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene
c19early.org, c19early.org (B), vitamin D
c19early.org (C), etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that
treated patients are more likely to be from affluent neighborhoods, be more
health-conscious, and have better access to care.
Therefore, these kind of studies may
overestimate the efficacy of treatments.
Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid
Hoertel. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy.
Black box warning. The FDA notes that
"severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid" FDA.
This study is excluded in the after exclusion results of meta
analysis:
only a fraction of eligible patients received treatment and these patients may be more likely to follow other recommendations, receive additional care, and more more likely to use additional untracked treatments such as vitamin D and nasal/oral hygiene.
|
risk of death, 95.2% lower, RR 0.05, p = 0.002, treatment 0 of 1,130 (0.0%), control 10 of 1,130 (0.9%), NNT 113, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching, day 30.
|
|
risk of progression, 39.2% lower, RR 0.61, p < 0.001, treatment 89 of 1,130 (7.9%), control 163 of 1,130 (14.4%), NNT 15, odds ratio converted to relative risk, combined ER/hospitalization/death, propensity score matching, day 30.
|
|
risk of progression, 32.9% lower, HR 0.67, p = 0.003, treatment 89 of 1,130 (7.9%), control 163 of 1,130 (14.4%), combined ER/hospitalization/death, propensity score matching, Kaplan–Meier, day 30.
|
|
risk of hospitalization, 56.5% lower, RR 0.44, p = 0.02, treatment 10 of 1,130 (0.9%), control 23 of 1,130 (2.0%), NNT 87, odds ratio converted to relative risk, propensity score matching, day 30.
|
|
Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates
|
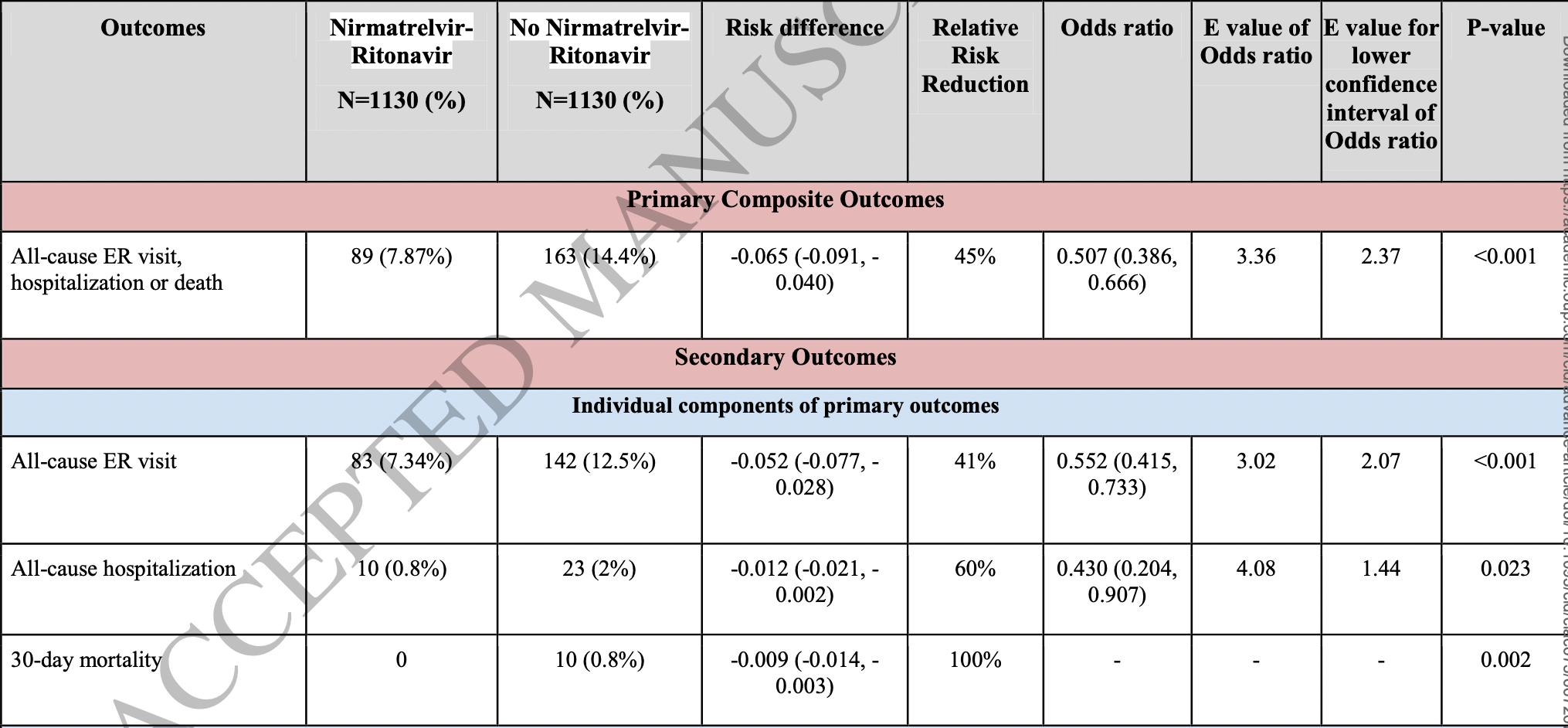
Ganatra et al., 20 Aug 2022, retrospective, USA, peer-reviewed, mean age 57.6, 9 authors, study period 1 December, 2021 - 18 April, 2022.
Contact:
sarju.ganatra@lahey.org.
Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19
MD Sarju Ganatra, MD, MSc Sourbha S Dani, MD Javaria Ahmad, MD Ashish Kumar, MD Jui Shah, MD, MPH George M Abraham, MD Daniel P Mcquillen, MD Robert M Wachter, MD Paul E Sax
doi:10.1093/cid/ciac673/6672670
Background Treatment of coronavirus disease-2019 (Covid-19) with nirmatrelvir plus ritonavir (NMV-r) in high-risk non-hospitalized unvaccinated patients reduced the risk of progression to severe disease. However, the potential benefits of NMV-r among vaccinated patients are unclear.
Methods We conducted a comparative retrospective cohort study using the TriNetX research network. Patients ≥18 years of age who were vaccinated and subsequently developed Covid-19 between December 1, 2021, and April 18, 2022, were included. Cohorts were developed based on the use of NMV-r within five days of diagnosis. The primary composite outcome was all-cause emergency room (ER) visit, hospitalization, or death at a 30-days follow-up. Secondary outcomes included individual components of primary outcomes, multisystem symptoms, Covid-19 associated complications, and diagnostic test utilization.
Results After propensity score matching, 1,130 patients remained in each cohort. A primary composite outcome of all-cause ER visits, hospitalization, or death in 30 days occurred in 89 (7.87%) patients in the NMV-r cohort as compared to 163 (14.4%) patients in the non-NMV-r cohort (OR 0.5, CI 0.39-0.67; p<0.005) consistent with 45% relative risk reduction. A significant reduction in multisystem symptom burden and subsequent complications such as lower respiratory tract infection, cardiac arrhythmia, and diagnostic radiology testing were noted in NMV-r treated patients. There was no apparent increase serious complications between days 10 to 30.
Conclusion Treatment with NMV-r in non-hospitalized vaccinated patients with Covid-19 was associated with a reduced likelihood of emergency room visits, hospitalization, or death. Complications and overall resource utilization were also decreased.
References
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Commissioner, The, Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 that Retains Activity Against Omicron Variant
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Mensah, Lacy, Stowe, Disease severity during SARS-COV-2 reinfection: a nationwide study, Journal of Infection
Paxlovid, added to PANORAMIC study -PANORAMIC
Pfizer, An Interventional Efficacy And Safety, Phase 2/3, Double-Blind, 2 Arm Study To Investigate Orally Administered Pf
Tenforde, Self, Adams, Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity, JAMA
Vanderweele, Ding, Sensitivity Analysis in Observational Research: Introducing the E-Value, Ann Intern Med