Efficacy is variant dependent. In Vitro studies suggest a lack of efficacy for many omicron variants Haars, Liu, Pochtovyi, Sheward, Tatham, VanBlargan. ADE shown In Vitro Shimizu. mAb use may create new variants that spread globally Focosi, Leducq, and may be associated with prolonged viral loads, clinical deterioration, and immune escape Choudhary, Günther, Leducq.
Recent:Iustila-Maran Wilcock.
Casirivimab/imdevimab was adopted
in 42 countries.
Submit updates/corrections .
May 15 |
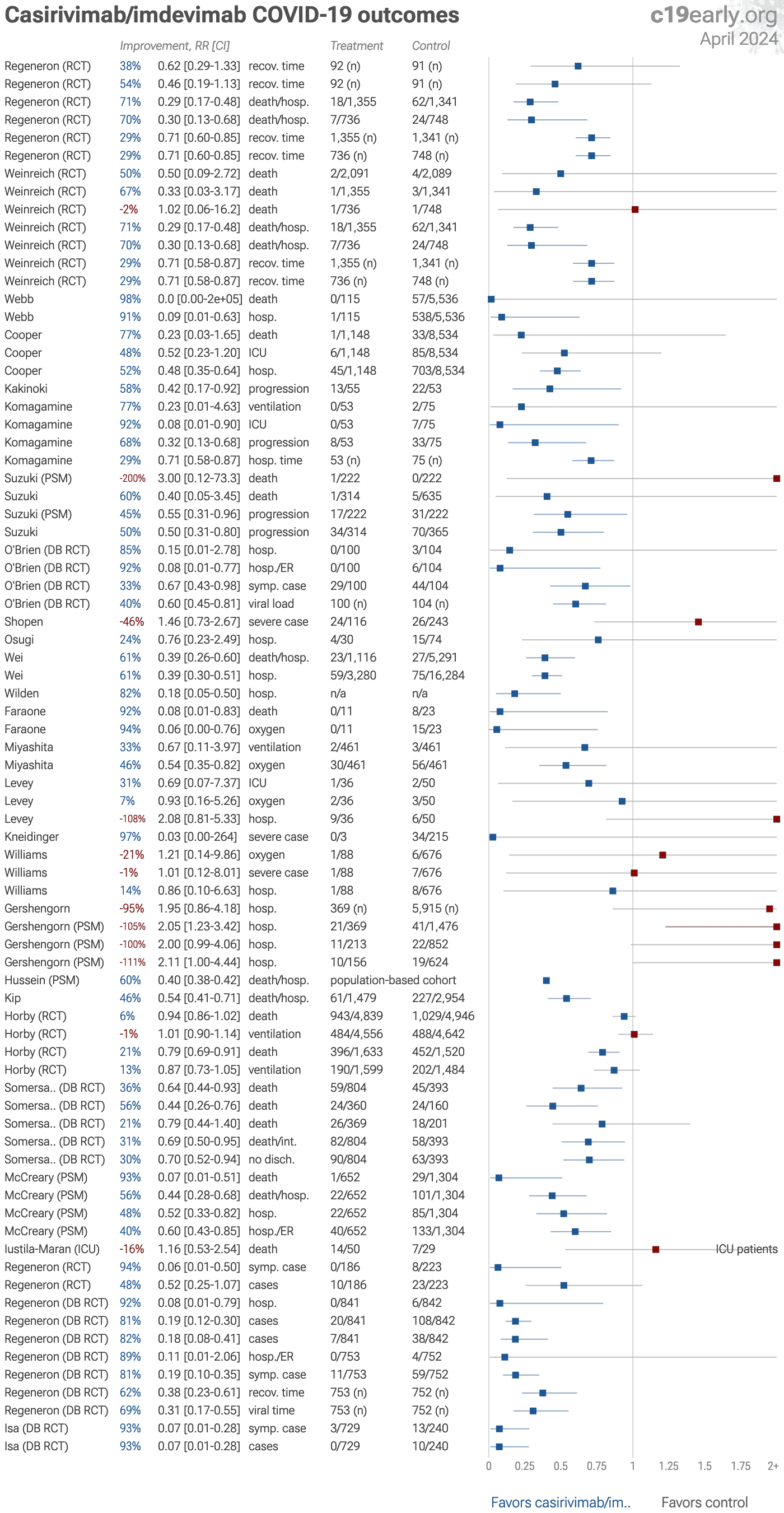
Casirivimab/imdevimab for COVID-19: real-time meta analysis of 28 studies | |
| Statistically significant lower risk is seen for hospitalization, progression, recovery, cases, and viral clearance. 20 studies from 14 independent teams in 4 countries show significant improvements. Meta analysis using the most serious o.. | ||
Apr 5 |
et al., Research Square, doi:10.21203/rs.3.rs-4090027/v1 | Course of inflammation and infection markers differ in ICU patients with severe COVID-19 under casirivimab- and/or tocilizumab application: an observational study |
| 16% higher mortality (p=0.8). Retrospective 95 ICU patients showing no significant difference in mortality with casirivimab/imdevimab. There was significantly higher mortality with tocilizumab. | ||
Jan 26 |
et al., JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044 | Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population |
| Analysis of Medicare beneficiaries in 2022 showing that outpatient COVID-19 treatments like antivirals and monoclonal antibodies were disproportionately used by patients at lower risk of severe infection and outcomes. Retrospective studie.. | ||
Nov 27 2023 |
et al., Heliyon, doi:10.1016/j.heliyon.2023.e22839 | Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial |
| Systematic review and meta analysis showing lower mortality and progression, and improved viral load with casirivimab/imdevimab treatment. | ||
Nov 27 2023 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad500.615 | COVID-19 Monoclonal Antibody Use at a Stand Alone Children’s Hospital |
| 91% higher hospitalization (p=0.5) and 91% higher progression (p=0.38). Retrospective 262 pediatric patients referred for COVID-19 monoclonal antibody treatment, 134 treated (74% receiving casirivimab/imdevimab), showing higher ER visits and hospitalization with treatment, without statistical significance. Au.. | ||
Nov 23 2023 |
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiad523 | Spike protein genetic evolution in patients at high-risk of severe COVID-19 treated by monoclonal antibodies |
| Prospective study of 264 high-risk COVID-19 patients treated with monoclonal antibodies. Tixagevimab/cilgavimab was associated with 5 times higher risk of emergence of mutations. Treatment with sotrovimab was linked to mutations associate.. | ||
Sep 28 2023 |
et al., Vaccines, doi:10.3390/vaccines11101533 | In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 |
| In Vitro study showing sharply reduced neutralization of SARS-CoV-2 variants XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 with monoclonal antibodies cilgavimab, tixagevimab, imdevimab, etsevimab, casirivim.. | ||
Sep 27 2023 |
et al., Microorganisms, doi:10.3390/microorganisms11102417 | Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023 |
| Analysis of 7,950 SARS-CoV-2 samples from central Sweden collected between March 2022 and May 2023 tracking the prevalence of omicron sublineages and mutations in the spike protein conferring resistance to monoclonal antibodies over time... | ||
Aug 10 2023 |
et al., Drug Resistance Updates, doi:10.1016/j.drup.2023.100991 | Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment |
| Review of reports of treatment-emergent resistance to COVID-19 monoclonal antibodies (mAbs), showing that some post-mAb treatment mutations appeared to spread globally soon after the mAb was introduced, raising concerns about transmission.. | ||
Jun 10 2023 |
et al., Value in Health, doi:10.1016/j.jval.2023.03.2056 | Variation in Demographic Characteristics, Socioeconomic Status, Clinical Presentation and Selected Treatments in Mortality Among Patients with a Diagnosis of COVID-19 in the United States |
| Retrospective analysis of mortality for COVID-19 patients in the USA. Authors do not provide adjusted results, preventing any strong evidence. However it is notable that, despite comparable treatment frequencies, the mortality for patient.. | ||
Apr 4 2023 |
et al., Annals of Internal Medicine, doi:10.7326/M22-1286 | Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 |
| 46% lower combined mortality/hospitalization (p<0.0001). Retrospective 2,571 patients treated with mAbs in the USA, and 5,135 control patients, showing lower combined mortality/hospitalization for bamlanivimab, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, and bebtelovimab, with s.. | ||
Dec 19 2022 |
et al., BMJ Open, doi:10.1136/bmjopen-2022-064953 | Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database |
| 60% lower combined mortality/hospitalization (p<0.0001). Retrospective 73,759 outpatients treated with casirivimab/imdevimab, showing lower mortality with treatment. This result is subject to potentially substantial confounding by indication - patients with more severe cases may be more likely .. | ||
Dec 2 2022 |
et al., PLOS ONE, doi:10.1371/journal.pone.0278770 | The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants |
| 95% higher hospitalization (p=0.09). Retrospective 2,083 outpatients in the USA, showing higher risk of hospitalization with casirivimab/imdevimab, without statistical significance. There may be significant unadjusted confounding by indication. | ||
Nov 17 2022 |
et al., bioRxiv, doi:10.1101/2022.11.17.516888 | Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies |
| In Vitro study suggesting a lack of efficacy for casirivimab/imdevimab with BA.2.75.2 and BQ.1.1. | ||
Oct 15 2022 |
et al., American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxac295 | The impact of COVID-19 monoclonal antibodies on clinical outcomes: A retrospective cohort study |
| 61% higher mortality (p=0.4), 51% higher ICU admission (p=0.5), 49% lower hospitalization (p<0.0001), and 39% lower progression (p<0.0001). PSM retrospective 1,344 patients in the USA, showing lower hospitalization with monoclonal antibody treatment. Authors combine patients treated with bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab. | ||
Sep 16 2022 |
et al., Scientific Reports, doi:10.1038/s41598-022-19993-w | Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells |
| In Vitro study showing that casirivimab/imdevimab may induce antibody-dependent enhancement (ADE) within a specific concentration range. No ADE was observed for sotrovimab. | ||
Sep 12 2022 |
et al., American Journal of Obstetrics and Gynecology, doi:10.1016/j.ajog.2022.09.017 | Effectiveness of REGEN-COV combination monoclonal antibody infusion to reduce risk of COVID-19 hospitalization in pregnancy: A retrospective cohort study |
| 21% higher need for oxygen therapy (p=0.87), 1% higher severe cases (p=0.99), and 14% lower hospitalization (p=0.9). Retrospective 764 pregnant patients with COVID-19 in the USA, 88 treated with casirivimab/imdevimab, showing no significant difference in outcomes. | ||
Sep 9 2022 |
et al., Infection, doi:10.1007/s15010-022-01914-8 | Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study |
| 97% lower severe cases (p=0.45). Retrospective 218 COVID+ lung transplant patients in Germany, showing no significant difference in severe cases with early casirivimab/imdevimab use. | ||
Jul 11 2022 |
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2022.07.002 | Timing of REGEN-COV administration and progression to severe COVID-19 |
| Retrospective 342 patients in Japan, showing significantly greater efficacy of casirivimab/imdevimab with earlier treatment. The proportion of patients progressing to severe COVID-19 increased daily from symptom onset and increased sharpl.. | ||
Jun 4 2022 |
et al., American Journal of Obstetrics & Gynecology MFM, doi:10.1016/j.ajogmf.2022.100673 | Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic |
| 108% higher hospitalization (p=0.15). Retrospective 86 pregnant COVID-19 patients, 36 treated with casirivimab/imdevimab, showing no significant difference in COVID-19 outcomes with treatment. | ||
May 26 2022 |
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2022.05.012 | Clinical efficacy of casirivimab-imdevimab antibody combination treatment in patients with COVID-19 Delta variant |
| 46% lower need for oxygen therapy (p=0.004). Retrospective 461 patients treated with casirivimab/imdevimab in Japan, and 461 matched controls, showing lower oxygen requirements with treatment. | ||
May 5 2022 |
et al., Research Square, doi:10.21203/rs.3.rs-1170976/v1 | REGEN-COV antibody cocktail (casirivimab/imdevimab) for the treatment of inpatients with early hospital-acquired COVID-19: a single center experience |
| 92% lower mortality (p=0.03) and 94% lower need for oxygen therapy (p=0.02). Retrospective 34 patients with hospital-acquired COVID-19, showing lower mortality and oxygen requirements with early casirivimab/imdevimab treatment. | ||
Mar 31 2022 |
et al., Journal of the National Comprehensive Cancer Network, doi:10.6004/jnccn.2021.7309 | Real World Outcomes of Cancer Patients With SARS-CoV-2 Infection Receiving Monoclonal Antibodies |
| 82% lower hospitalization (p=0.004). Retrospective 395 patients in the USA receiving casirivimab/imdevimab or bamlanivimab, showing lower risk of hospitalization with treatment, statistically significant for casirivimab/imdevimab. | ||
Feb 28 2022 |
et al., medRxiv, doi:10.1101/2022.02.28.22270796 | Real-world Effectiveness of Casirivimab and Imdevimab in Patients With COVID-19 in the Ambulatory Setting: An Analysis of Two Large US National Claims Databases |
| 61% lower combined mortality/hospitalization (p<0.0001) and 61% lower hospitalization (p<0.0001). Retrospective 4,396 casirivimab/imdevimab patients in the USA, showing lower combined mortality/hospitalization (CDM database) and lower hospitalization (PMTX+ database) with treatment. | ||
Feb 16 2022 |
et al., bioRxiv, doi:10.1101/2022.02.15.480166 | SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies |
| In Vitro study showing that omicron BA.2 evades all monoclonal antibodies tested, including sotrovimab and tixagevimab/cilgavimab which retained activity for omicron BA.1. | ||
Feb 3 2022 |
et al., Cureus, doi:10.7759/cureus.21882 | Clinical Prognosis of Patients With Mild COVID-19 Treated With Casirivimab/Imdevimab in Japan |
| 24% lower hospitalization (p=0.65). Retrospective 104 outpatients in Japan, 30 treated with casirivimab/imdevimab, showing no significant difference in hospitalization. | ||
Jan 31 2022 |
et al., medRxiv, doi:10.1101/2022.01.29.22270090 | Doubtful Clinical Benefit of Casirivimab-Imdevimab Treatment for Disease Severity Outcome of High-Risk Patients with SARS-CoV-2 Delta Variant Infection |
| 46% higher severe cases (p=0.26). Retrospective 359 COVID+ patients in Israel, 116 treated with casirivimab/imdevimab, showing no significant difference with treatment in multivariable analysis. | ||
Jan 24 2022 |
et al., bioRxiv, doi:10.1101/2022.01.23.477397 | Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice |
| K18-hACE2 mouse study showing that casirivimab/imdevimab was not effective for omicron at doses 2x higher than those effective for previous variants. | ||
Jan 14 2022 |
et al., JAMA, doi:10.1001/jama.2021.24939768 | Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial |
| 85% lower hospitalization (p=0.25), 92% fewer combined hospitalization/ER visits (p=0.03), 33% fewer symptomatic cases (p=0.04), and 40% improved viral clearance (p=0.001). RCT 204 asymptomatic COVID+ patients, 100 treated with subcutaneous casirivimab/imdevimab, showing lower development of symptoms, lower hospitalization, and faster viral clearance with treatment. Study conducted prior to widespread circul.. | ||
Dec 21 2021 |
et al., medRxiv, doi:10.1101/2021.12.19.21268078 | Real-world clinical outcomes of treatment with casirivimab-imdevimab among patients with mild-to-moderate coronavirus disease 2019 during the Delta variant pandemic |
| 45% lower progression (p=0.02). Retrospective 949 patients in Japan, 314 treated with casirivimab/imdevimab showing significantly lower risk of deterioration with treatment. | ||
Dec 20 2021 |
et al., bioRxiv, doi:10.1101/2021.12.19.473354 | Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron) |
| In Vitro study showing that omicron is substantially resistant to neutralization by monoclonal antibodies REGN10933, REGN10987, Ly-CoV016 and Ly-CoV555. S309 (the parent of Sotrovimab) had only 2-fold loss in potency. | ||
Dec 19 2021 |
et al., Journal of General and Family Medicine, doi:10.1002/jgf2.516 | The effect of casirivimab with imdevimab on disease progression in nonsevere COVID-19 patients in a single hospital in Japan |
| 92% lower ICU admission (p=0.04), 68% lower progression (p=0.006), and 29% shorter hospitalization (p=0.001). Combined retrospective/prospective study in Japan with 53 casirivimab/imdevimab patients and 75 control patients, showing significantly lower progression with treatment. | ||
Dec 17 2021 |
et al., bioRxiv, doi:10.1101/2021.12.15.472828 | An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies |
| In vitro study (Vero-TMPRSS2 and Vero-hACE2-TMPRSS2) showing complete loss of inhibitory activity for B.1.1.529 omicron with LY-CoV555, LY-CoV016, REGN10933, REGN10987, and CT-P59, ~12-fold decrease for COV2-2196/COV2-2130, and minimal ch.. | ||
Dec 15 2021 |
et al., bioRxiv, doi:10.1101/2021.12.14.472719 | Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2 |
| In Vitro study (Vero-E6-TMPRSS2) showing 18 of 19 monoclonal antibodies were no longer effective or significantly impaired with B.1.1.529 omicron. | ||
Dec 1 2021 |
et al., medRxiv, doi:10.1101/2021.11.30.21266756 | Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19 |
| 93% lower mortality (p=0.009), 56% lower combined mortality/hospitalization (p=0.0003), 48% lower hospitalization (p=0.005), and 40% fewer combined hospitalization/ER visits (p=0.003). Prospective study comparing subcutaneous and intravenous casirivimab/imdevimab, and comparing to a PSM matched control set, showing significantly lower mortality and hospitalization with treatment. Controls were matched with EUA-eligible .. | ||
Nov 16 2021 |
et al., medRxiv, doi:10.1101/2021.11.10.21265889 | Repeat Subcutaneous Administration of REGEN-COV® in Adults is Well-Tolerated and Prevents the Occurrence of COVID-19 |
| 93% fewer symptomatic cases (p=0.002) and 93% fewer cases (p=0.002). RCT 969 patients, 729 treated with monthly subcutaneous casirivimab/imdevimab, showing significantly lower risk of COVID-19 with treatment. There were no grade 3 injection site reactions or hypersensitivity reactions. Slightly more TEAEs .. | ||
Nov 8 2021 |
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiac320 (date from preprint) | Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19 |
| 36% lower mortality (p=0.02), 31% lower combined mortality/intubation (p=0.03), and 30% higher hospital discharge (p=0.02). RCT 1,336 hospitalized patients with symptom onset <=10 days on low-flow or no supplemental oxygen, showing lower mortality with treatment. Cohorts 2&3 were paused mid-trial due to increased deaths in the treatment arm and these results w.. | ||
Nov 8 2021 |
, Press Release | New phase 3 analyses show that a single dose of REGEN-COV® (casirivimab and imdevimab) provides long-term protection against COVID-19 |
| 92% lower hospitalization (p=0.03), 81% fewer cases (p<0.0001), 89% fewer combined hospitalization/ER visits (p=0.06), and 81% fewer symptomatic cases (p<0.0001). Long-term results for PEP RCT NCT04452318, with 841 baseline seronegative casirivimab/imdevimab patients and 842 placebo patients, showing significantly lower cases with treatment. | ||
Nov 4 2021 |
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.01.067 (date from preprint) | Impact of Antibody Cocktail Therapy Combined with Casirivimab and Imdevimab on Clinical Outcome for Covid-19 patients in A Real-Life Setting: A Single Institute Analysis |
| 58% lower progression (p=0.05). Retrospective 55 patients in Japan treated a median of 3 days from symptom onset with casirivimab/imdevimab, and 53 control patients, showing lower risk of further treatment including oxygen or antivirals. | ||
Nov 2 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-995033/v1 | Antibody escape and global spread of SARS-CoV-2 lineage A.27 |
| Anaysis of antibody escape showing variant A.27 completely escaped neutralization with LY-COV555 and partially with REGN10987. B.1.617.2 escaped these antibodies in a similar manner, suggesting that L452R facilitates the escape. Authors n.. | ||
Oct 31 2021 |
et al., EClinicalMedicine, doi:10.1016/j.eclinm.2021.101102 | Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19 |
| 75% lower mortality (p=0.37), 29% lower ICU admission (p=0.77), and 67% lower hospitalization (p=0.001). Retrospective 696 patients treated with casirivimab/imdevimab, and 696 matched controls, showing lower hospitalization with treatment. Authors only included patients with documented followup, which is likely to disproportionately bias the.. | ||
Oct 20 2021 |
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiab570 (date from preprint) | Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities |
| 89% lower ICU admission (p=0.16), 85% lower need for oxygen therapy (p<0.0001), and 75% lower hospitalization (p<0.0001). Retrospective 1,395 vaccinated COVID-19 cases, showing significantly lower hospitalization and oxygen supplementation with monoclonal antibody treatment, primarily casirivimab-imdevimab. Hospitalization was significantly associated with.. | ||
Oct 8 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab512 | Real-world Assessment of 2,879 COVID-19 Patients Treated with Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study |
| 77% lower mortality (p=0.18), 48% lower ICU admission (p=0.14), and 52% lower hospitalization (p<0.0001). Retrospective 2,879 patients and matched controls in the USA, showing significantly lower mortality and hospitalization with bamlanivimab, bamlanivimab/etesevimab, and casirivimab/imdevimab. There was significantly lower hospitalization w.. | ||
Jun 23 2021 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab331 | Real-World Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients with Early COVID-19 |
| 91% lower hospitalization (p=0.0003). Retrospective 115 patients treated with casirivimab/imdevimab showing lower mortality, hospital admission, and emergency department visits with treatment. Authors incorrectly state that "no other COVID-19 therapies for ambulatory pat.. | ||
Jun 16 2021 |
et al., The Lancet, doi:10.1016/S0140-6736(22)00163-5 (date from preprint) | Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial |
| 6% lower mortality (p=0.16) and 1% higher ventilation (p=0.88). RCT 9,785 hospitalized patients in the UK showing lower mortality with casirivimab/imdevimab, with statistical significance reached for baseline seronegative patients. | ||
May 21 2021 |
et al., NEJM, doi:10.1056/NEJMoa2108163 (date from preprint) | REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19 |
| 71% lower combined mortality/hospitalization (p=0.001) and 29% faster recovery (p=0.001). RCT 4,057 outpatients with >=1 risk factor for severe disease, showing significantly lower combined hospitalization/death, and significantly faster recovery with treatment. Median time from onset of symptoms 3 days. | ||
Apr 12 2021 |
et al., NEJM, doi:10.1056/NEJMoa2109682 (press release 4/12/21) | Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19 |
| 89% fewer combined hospitalization/ER visits (p=0.06), 81% fewer symptomatic cases (p<0.0001), 62% faster recovery (p=0.0001), and 69% faster viral clearance (p=0.0001). Prophylaxis trial reporting lower hospitalization/ER and symptomatic cases, and faster recovery with 1,200mg subcutaneous casirivimab with imdevimab. The same trial has updated results available in [c19regn.com]. NCT04452318. | ||
Mar 23 2021 |
, Press Release | New phase III data shows investigational antibody cocktail casirivimab and imdevimab reduced hospitalisation or death by 70% in non-hospitalised patients with COVID-19 |
| 71% lower combined mortality/hospitalization (p<0.0001) and 29% faster recovery (p=0.0001). Press release for new phase III data showing lower hospitalization/mortality, and faster symptom resolution among the subset of patients with at least one risk factor. Some variants may escape antibodies [cell.com]. | ||
Jan 26 2021 |
, Press Release | Regeneron Reports Positive Interim Data with REGEN-COV™ Antibody Cocktail used as Passive Vaccine to Prevent COVID-19 |
| 94% fewer symptomatic cases (p=0.009) and 48% fewer cases (p=0.07). Interim results of REGEN-COV prophylaxis showing 100% prevention of symptomatic infection (8/223 placebo vs. 0/186 REGEN-COV), and approximately 50% lower overall rates of infection (symptomatic and asymptomatic) (23/223 placebo vs. 10/18.. | ||
Sep 29 2020 |
, Press Release | Regeneron's REGN-COV2 antibody cocktail reduced viral levels and improved symptoms in non-hospitalized COVID-19 patients |
| 38% faster recovery (p=0.22). Analysis of the first 275 patients in a trial of the REGN-COV2 antibody cocktail showing reductions in viral load and the time to alleviate symptoms in non-hospitalized patients with COVID-19. Greatest improvements were seen with patients.. | ||
Aug 21 2020 |
et al., Science, 21 Aug 2020, 369:6506, 1014-1018, doi:10.1126/science.abd0831 | Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies |
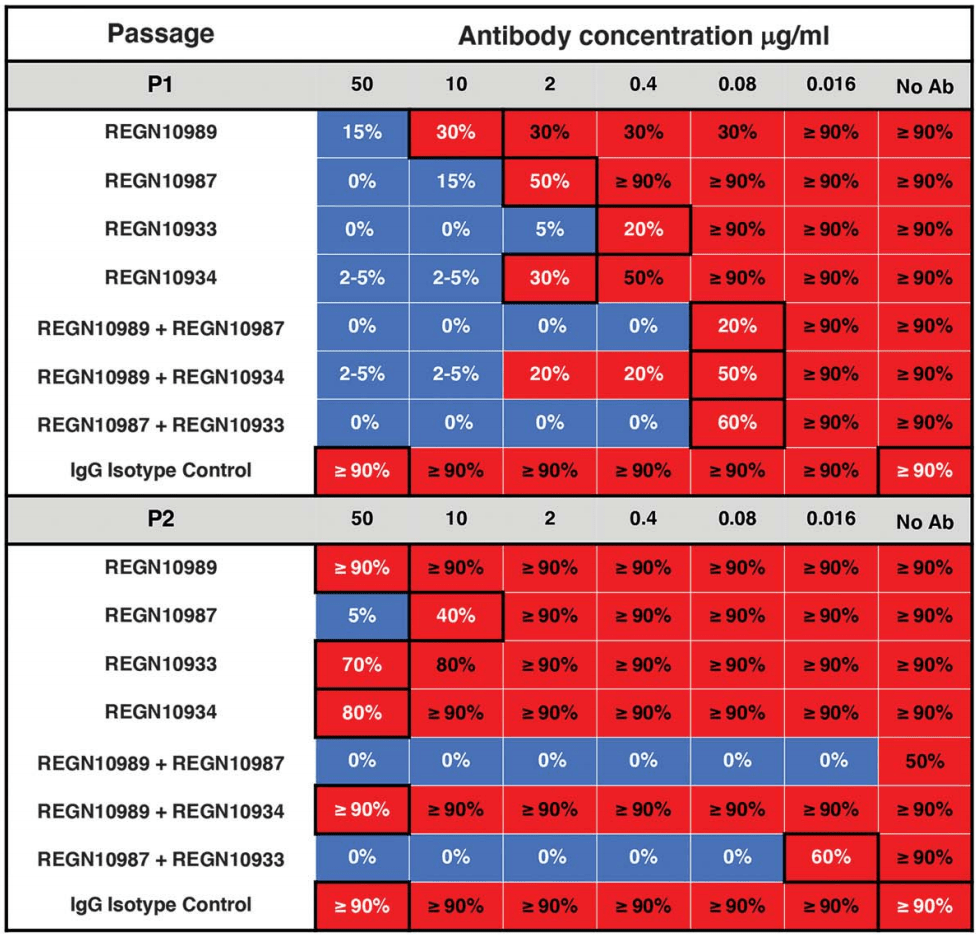
| In Vitro study showing that, under pressure from individual antibodies, mutant viruses were rapidly selected that evaded the blocking function of all individual antibodies tested, including antibodies that potently bind to highly-conserve.. | ||
Aug 21 2020 |
et al., Science, 21 Aug 2020, 369:6506, 1010-1014, doi:10.1126/science.abd0827 | Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail |
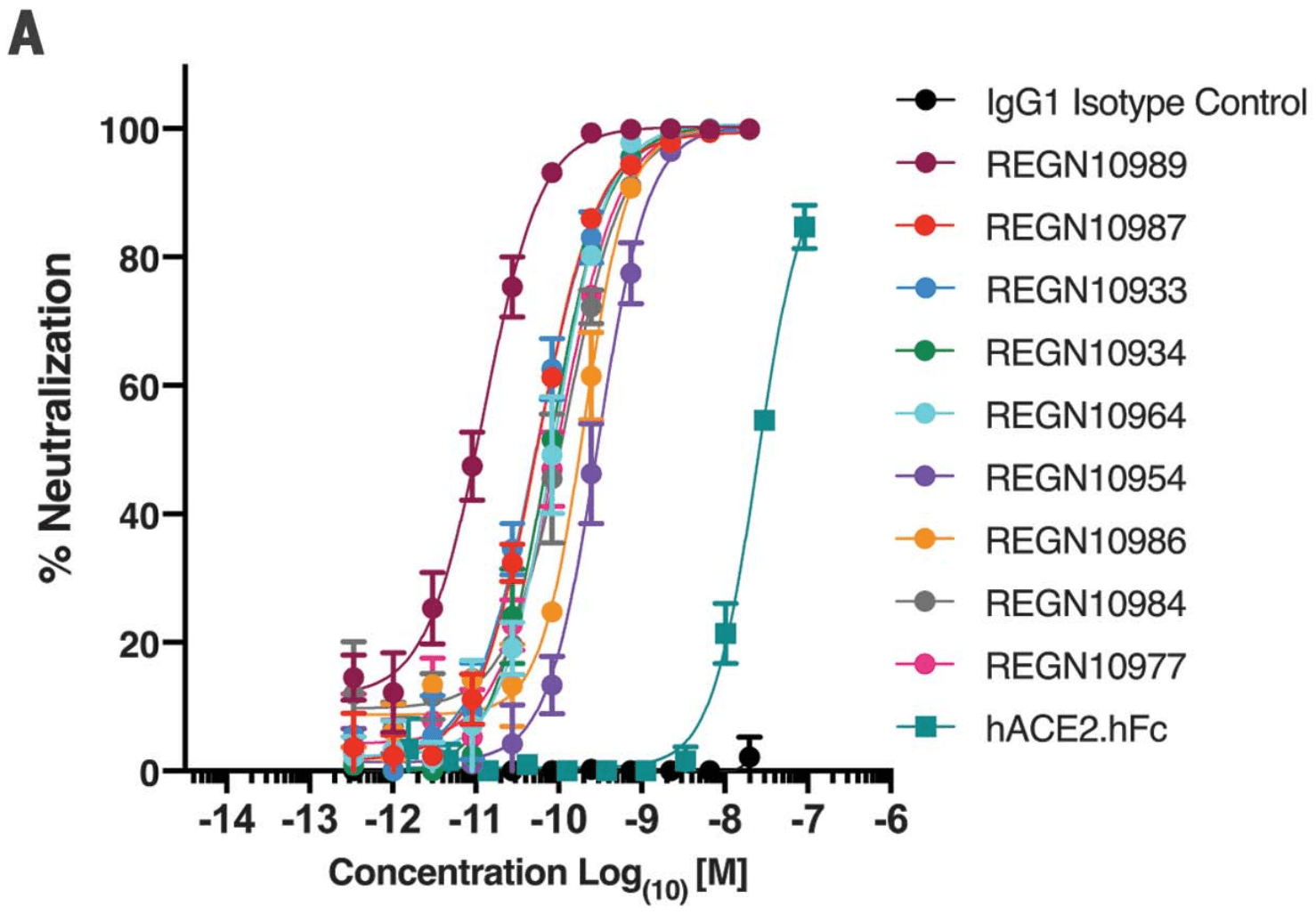
| Study using humanized mice and blood samples from recovered COVID-19 patients to generate antibodies targeting multiple different regions of the critical receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. The spike protein on .. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. FLCCC and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.