Pfizer has denied access to Paxlovid for independent RCTs Ledford. Pfizer RCTs report very good results, while non-Pfizer RCTs show relatively poor results Liu, Yu. Hoertel find that >50% of patients that died had a contraindication for Paxlovid. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that "severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid" FDA. Population studies often do not account for the different expected outcomes for the class of patients that seek out and receive early treatment.
Paxlovid is a combination of nirmatrelvir and ritonavir. Nirmatrelvir is a first generation SARS-CoV-2 3CL protease inhibitor Bischof. Ritonavir is a HIV drug used to boost the levels of nirmatrelvir in the body by inhibiting its metabolism.
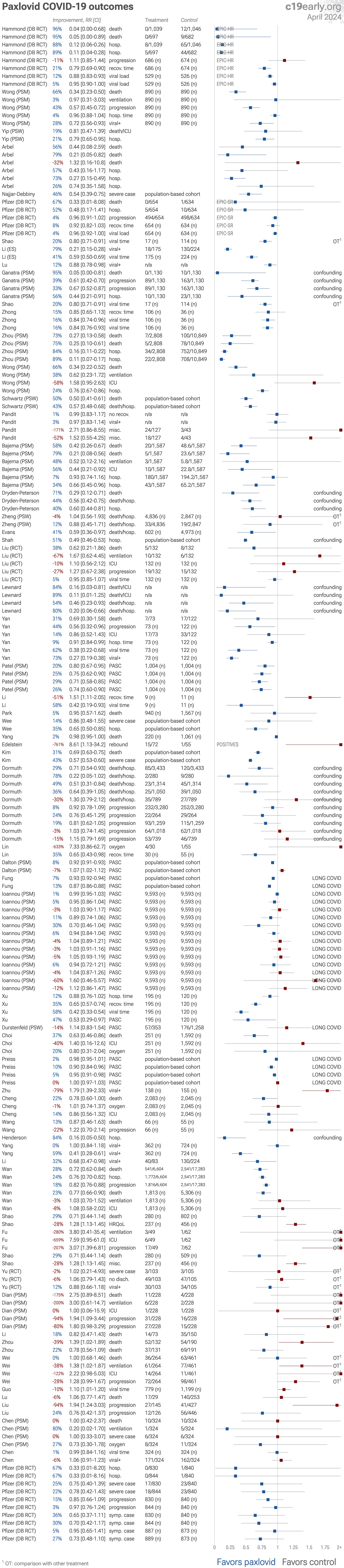
Recent:Chen Lewandowski Hammond Malden Yang Schreiber Zhu.
Paxlovid has been officially adopted
in 43 countries.
Submit updates/corrections .
Apr 5 |
et al., Immunity, Inflammation and Disease, doi:10.1002/iid3.1232 | Nirmatrelvir and ritonavir combination against COVID‐19 caused by omicron BA.2.2 in the elderly: A single‐center large observational study |
| PSM retrospective 648 elderly COVID-19 patients showing no significant difference in time to viral clearance with paxlovid. However, in subgroup analysis of patients treated within 10 days of symptom onset, treatment was associated with f.. | ||
Apr 4 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2309003 | Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19 |
| Delayed publication for EPIC-SR showing no significant difference in time to sustained alleviation. Selected results were first made available in December 2021 [Pfizer]. | ||
Apr 2 |
et al., bioRxiv, doi:10.1101/2024.04.01.587566 | Distal Protein-Protein Interactions Contribute to SARS-CoV-2 Main Protease Substrate Binding and Nirmatrelvir Resistance |
| In Vitro and crystallographic study reveals that the L50F mutation in SARS-CoV-2 main protease (Mpro) can restore the reduced enzymatic activity caused by nirmatrelvir resistance mutations E166A/L167F through enhanced protein-protein inte.. | ||
Mar 29 |
et al., Infection and Drug Resistance, doi:10.2147/IDR.S443574 | Reduced Viral Shedding Time in High-Risk COVID-19 Patients Infected by Omicron and Treated with Paxlovid: A Real-World Study from China |
| Retrospective 3,159 high risk COVID-19 patients in China showing no significant difference for viral clearance with multivariable Cox regression, but significantly faster viral clearance with logistic regression. Cox results account for t.. | ||
Mar 29 |
et al., Scientific Reports, doi:10.1038/s41598-024-57633-7 | Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system |
| Retrospective 319,900 treatment-eligible COVID-19 patients showing relatively low use of paxlovid and significant socioeconomic disparities. Treated patients were more likely to be from affluent neighborhoods, be up to date on vaccination.. | ||
Mar 13 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2024.1765 | COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment |
| RCT showing high rates of viral and symptom rebound with both paxlovid and deuremidevir (VV116). There are multiple potential reasons, with one being the highly specific targets within viral replication (Mpro and RdRp respectively). Paxlo.. | ||
Mar 2 |
et al., Antiviral Research, doi:10.1016/j.antiviral.2024.105840 | The host-targeted antiviral drug Zapnometinib exhibits a high barrier to the development of SARS-CoV-2 resistance |
| In Vitro study showing that molnupiravir and paxlovid induced resistant variants in SARS-CoV-2 during serial passaging, while the host-directed antiviral zapnometinib did not. Authors found that molnupiravir did not lead to abrogated vira.. | ||
Feb 21 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkae042 | COVID-19 hospitalization risk after outpatient nirmatrelvir/ritonavir use, January to August 2022, North Carolina |
| 84% lower hospitalization (p=0.002). EHR retrospective 44, 671 patients with 4,948 receiving paxlovid, showing lower hospitalization with treatment. | ||
Feb 9 |
et al., Scientific Reports, doi:10.1038/s41598-024-53862-y | Comparison of azvudine, molnupiravir, and nirmatrelvir/ritonavir in adult patients with mild-to-moderate COVID-19: a retrospective cohort study |
| Retrospective 157 hospitalized mild-to-moderate COVID-19 patients showing no significant differences between azvudine, molnupiravir, and paxlovid for time to viral clearance and length of hospitalization. | ||
Feb 9 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2024.102468 | Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study |
| 13% lower mortality (p=0.67) and 22% higher progression (p=0.49). Retrospective 249 elderly patients with severe COVID-19, 128 treated with azvudine, 66 treated with paxlovid, and 55 receiving neither treatment, showing no significant differences for Ct value changes, progression, or survival for either.. | ||
Feb 9 |
et al., Kidney International Reports, doi:10.1016/j.ekir.2024.02.009 | Effectiveness of molnupiravir and nirmatrelvir–ritonavir in CKD patients with COVID-19 |
| 22% lower mortality (p=0.05), 1% higher need for oxygen therapy (p=0.96), and 14% lower ICU admission (p=0.5). Retrospective emulated target trial of hospitalized COVID-19 patients with chronic kidney disease in Hong Kong showing lower mortality with molnupiravir and paxlovid treatment. No significant reduction was found in ICU admission or ventil.. | ||
Jan 31 |
et al., The Journal of Infection in Developing Countries, doi:10.3855/jidc.18138 | Clinical characteristics and risk factors of non-mild outcomes in patients with Omicron variant COVID-19 in Shanghai, China |
| 79% worse viral clearance (p<0.0001). Retrospective 311 COVID-19 patients in China showing significantly slower viral clearance with paxlovid in multivariable analysis. | ||
Jan 26 |
et al., JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044 | Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population |
| Analysis of Medicare beneficiaries in 2022 showing that outpatient COVID-19 treatments like antivirals and monoclonal antibodies were disproportionately used by patients at lower risk of severe infection and outcomes. Retrospective studie.. | ||
Jan 24 |
et al., Research Square, doi:10.21203/rs.3.rs-3876022/v1 | Oral antivirals for COVID-19 among patients with cancer |
| Retrospective 67 cancer outpatients treated with nirmatrelvir/ritonavir or molnupiravir, compared to 56 untreated concurrent controls, reporting lower mortality with treatment. However, Figure 3 shows the opposite results for invasive mec.. | ||
Jan 22 |
et al., medRxiv, doi:10.1101/2024.01.20.24301525 | Effect of Paxlovid Treatment on Long COVID Onset: An EHR-Based Target Trial Emulation from N3C |
| 2% lower PASC (p=0.2). Retrospective study of 426,352 high-risk outpatients showing no significant difference in post-acute sequelae of COVID-19 (PASC) incidence with paxlovid treatment. Subgroup analysis showed benefits for cognitive and fatigue symptoms. The .. | ||
Jan 15 |
et al., Toxics, doi:10.3390/toxics12010073 | Ritonavir Has Reproductive Toxicity Depending on Disrupting PI3K/PDK1/AKT Signaling Pathway |
| In vitro study on boar spermatozoa showing that the HIV drug ritonavir (part of paxlovid and xiannuoxin) causes reproductive toxicity by disrupting the PI3K/PDK1/AKT signaling pathway. Ritonavir suppressed sperm functions including motili.. | ||
Jan 8 |
et al., SSRN, doi:10.2139/ssrn.4683854 | Comparative Efficacy of Combination Treatment with Nirmatrelvir-Ritonavir and Remdesivir Versus Remdesivir Monotherapy in Hospitalised COVID-19 Patients: A Target Trial Emulation Study |
| 37% lower mortality (p=0.004), 40% higher ICU admission (p=0.78), and 20% lower need for oxygen therapy (p=0.66). Retrospective 1,843 hospitalized COVID-19 patients in Hong Kong showing lower mortality with paxlovid. All patients received remdesivir. No significant difference was found for ICU admission or ventilatory support. | ||
Jan 4 |
et al., Journal of Medical Virology, doi:10.1002/jmv.29333 | Association of nirmatrelvir for acute SARS‐CoV‐2 infection with subsequent Long COVID symptoms in an observational cohort study |
| 14% higher PASC (p=0.4). Retrospective 4,684 COVID+ patients mostly in the USA, 988 treated with paxlovid, showing higher risk of long COVID with treatment, without statistical significance. | ||
Dec 31 2023 |
, E., Aging and disease, doi:10.14336/AD.2023.0318 | Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments |
| Review focusing on 3CL protease inhibitors. First generation inhibitors like paxlovid and simnotrelvir require boosting with ritonavir, which can cause drug-drug interactions and other issues. Second generation inhibitors like ensitrelvir.. | ||
Dec 22 2023 |
et al., Medicine, doi:10.1097/MD.0000000000036714 | Impact of Paxlovid on in-hospital outcomes and post-COVID-19 condition in adult patients infected with SARS-CoV-2 Omicron variant: A non-randomized controlled clinical trial |
| 12% shorter hospitalization (p=0.09), 35% faster recovery (p<0.0001), 58% faster viral clearance (p<0.0001), and 47% lower PASC (p=0.04). Prospective study of 320 COVID-19 patients infected with the SARS-CoV-2 Omicron variant in China, showing improved viral clearance and symptom resolution with 5 days of paxlovid treatment. Authors perform multivariable analysis for post-c.. | ||
Nov 30 2023 |
et al., Clinical Interventions in Aging, doi:10.2147/cia.s431271 | Factors Affecting Mortality in Elderly Hypertensive Hospitalized Patients with COVID-19: A Retrospective Study |
| 232% higher mortality (p=0.006). Retrospective 748 elderly hospitalized COVID-19 patients in China showing increased risk of death with paxlovid/molnupiravir. Multivariate analysis showed that paxlovid/molnupiravir was independently associated with higher in-hospital mor.. | ||
Oct 31 2023 |
et al., Annals of Internal Medicine, doi:10.7326/M23-1394 | Effectiveness of Nirmatrelvir–Ritonavir Against the Development of Post–COVID-19 Conditions Among U.S. Veterans |
| 1% lower PASC (p=0.75). Retrospective 9,593 veterans in the USA treated with paxlovid, matched to 9,593 untreated controls, showing no significant difference in post-COVID conditions across 31 different conditions. There was lower risk for the combination of 2 s.. | ||
Oct 23 2023 |
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2023.5099 | Nirmatrelvir and Molnupiravir and Post–COVID-19 Condition in Older Patients |
| 7% lower PASC (p<0.0001). Retrospective 51,658 paxlovid patients in the USA showing a small reduction in long COVID with treatment. Confounding is likely significant as below, and may eliminate the benefit. Results specific to the COVID-19 code should be closer to.. | ||
Oct 21 2023 |
et al., Heliyon, doi:10.1016/j.heliyon.2023.e21387 | Clinical characteristics, outcomes, and risk factors of SARS-CoV-2 breakthrough infections among 572 fully vaccinated (BBIBP-CorV) hospitalized patients |
| 94% higher progression (p=0.007). Retrospective 572 fully vaccinated hospitalized patients in China, showing higher risk with paxlovid use. The composite outcome included intubation, non-invasive respiratory support, ICU admission, and all-cause death. Details for analysi.. | ||
Oct 18 2023 |
et al., Infection and Drug Resistance, doi:10.2147/IDR.S430101 | Clinical Characteristics of Severe COVID-19 Patients During Omicron Epidemic and a Nomogram Model Integrating Cell-Free DNA for Predicting Mortality: A Retrospective Analysis |
| 6% higher mortality (p=0.84). Retrospective 282 severe COVID-19 patients, showing no significant difference in mortality with paxlovid in unadjusted results. | ||
Oct 18 2023 |
et al., BMC Infectious Diseases, doi:10.1186/s12879-023-08620-2 | In-hospital adverse outcomes and risk factors among chronic kidney disease patients infected with the omicron variant of SARS-CoV-2: a single-center retrospective study |
| 10% slower viral clearance (p=0.04). Retrospective 1,978 hospitalized patients in China, showing slower viral clearance with Paxlovid. Authors note improved results in the subgroup of non-severe patients with CKD. | ||
Oct 13 2023 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2023.1274294 | Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching |
| no change in mortality (p=1), 38% higher ventilation (p=0.04), 122% higher ICU admission (p=0.05), and 28% higher progression (p=0.07). PSM retrospective 725 hospitalized COVID-19 patients in China compared the effectiveness and safety of the oral antivirals azvudine and paxlovid. There was no significant difference in the risk of disease progression between groups, but a.. | ||
Oct 12 2023 |
et al., Frontiers in Microbiology, doi:10.3389/fmicb.2023.1280026 | Secondary pulmonary infection and co-infection in elderly COVID-19 patients during the pandemics in a tertiary general hospital in Beijing, China |
| 39% higher mortality (p=0.04). Retrospective 322 hospitalized patients ≥65 in China, showing higher mortality with paxlovid use. Details for analysis of confounding are not provided and authors note use may have been higher for more severe patients. The results for pax.. | ||
Oct 11 2023 |
et al., IDWeek 2023 | Use of Paxlovid for Treatment of Acute COVID-19 and Occurrence of Post-COVID Conditions among Children and Adults at High-Risk for Severe COVID-19, April 1 - December 31, 2022 |
| 8% lower PASC (p<0.0001). Retrospective 297,662 paxlovid patients in the USA, showing lower risk of post-COVID conditions for patients 50+, no significant difference for ages 18-49, and higher risk for age 12-17. | ||
Oct 5 2023 |
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.10.007 | Clinical outcomes of nirmatrelvir-ritonavir use in pregnant women during the Omicron wave of the coronavirus disease 2019 pandemic |
| 633% higher need for oxygen therapy (p=0.05) and 35% faster recovery (p=0.04). Retrospective 85 pregnant patients in Taiwan, 30 treated with paxlovid, showing higher oxygen requirements, not quite reaching statistical significance (p=0.05), and faster recovery. Patients taking paxlovid for less than three consecutiv.. | ||
Oct 2 2023 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2023.36678 | Nirmatrelvir-Ritonavir and COVID-19 Mortality and Hospitalization Among Patients With Vulnerability to COVID-19 Complications |
| 29% lower combined mortality/hospitalization (p=0.02) and 8% lower progression (p=0.37). Retrospective 3,433 high-risk patients and matched controls in Canada showing lower mortality with paxlovid use. Patients were divided into four groups based on risk, with improved results as risk increased. Authors did not exclude all.. | ||
Sep 28 2023 |
et al., Vaccines, doi:10.3390/vaccines11101533 | In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 |
| In Vitro study showing sharply reduced neutralization of SARS-CoV-2 variants XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1 with monoclonal antibodies cilgavimab, tixagevimab, imdevimab, etsevimab, casirivim.. | ||
Sep 28 2023 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2023.1238713 | Differences in the severity and mortality risk factors for patients hospitalized for COVID-19 pneumonia between the early wave and the very late stage of the pandemic |
| 18% lower mortality (p=0.61). Retrospective 223 hospitalized patients in China, showing no significant difference in mortality with paxlovid in unadjusted results. | ||
Aug 31 2023 |
et al., Journal of Infection, doi:10.1016/j.jinf.2023.05.012 | Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities |
| 175% higher mortality (p=0.11), 200% higher ventilation (p=0.28), and 94% higher progression (p=0.03). Retrospective 2,118 hospitalized COVID-19 patients in China, showing improved results with azvudine vs. paxlovid. | ||
Aug 16 2023 |
et al., Phytomedicine, doi:10.1016/j.phymed.2023.155025 | Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial |
| 2% higher severe cases (p=1), 6% lower hospital discharge (p=0.78), and 12% improved viral clearance (p=0.33). RCT 312 hospitalized COVID-19 patients in China, showing no significant difference between paxlovid and Huashi Baidu treatment. Combination therapy showed improved results to either treatment alone. | ||
Aug 7 2023 |
et al., BMJ Open, doi:10.1136/bmjopen-2022-069176 | Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease |
| PANORAMIC protocol paper, published over 9 months after publication of the molnupiravir arm [Butler]. | ||
Jul 23 2023 |
et al., Microorganisms, doi:10.3390/microorganisms11071859 | Composite Interventions on Outcomes of Severely and Critically Ill Patients with COVID-19 in Shanghai, China |
| 29% lower mortality (p=0.16) and 28% worse results (p<0.0001). Retrospective 1,082 severely and critically ill COVID-19 patients in China showing lower 60 day mortality with azvudine. Mortality was also lower with paxlovid, but without statistical significance, and health related quality of life was.. | ||
Jul 10 2023 |
et al., Journal of Korean Medical Science, doi:10.3346/jkms.2023.38.e211 | Effectiveness of Paxlovid, an Oral Antiviral Drug, Against the Omicron BA.5 Variant in Korea: Severe Progression and Death Between July and November 2022 |
| 31% lower mortality (p<0.0001) and 43% lower severe cases (p<0.0001). Retrospective 1,936,925 COVID-19 patients in South Korea, showing lower mortality with paxlovid. | ||
Jun 27 2023 |
et al., medRxiv, doi:10.1101/2023.06.23.23288598 | SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy |
| 761% worse viral clearance (p=0.04). Prospective study of 127 COVID-19 patients in the USA showing higher risk of replication-competent virologic rebound with paxlovid treatment. Authors note that rebound substantially increases the duration of shedding of replication-compet.. | ||
Jun 26 2023 |
et al., Authorea, Inc., doi:10.22541/au.168777909.90198442/v1 | Comparison of the Different Medications for COVID-19 in Kidney Transplant Recipients |
| 280% higher ventilation (p=0.32), 659% higher ICU admission (p=0.04), and 207% higher progression (p=0.005). Retrospective 140 kidney transplant patients, showing higher risk of AKI with paxlovid compared with azvudine. There were more severe cases in the paxlovid group at baseline. | ||
Jun 22 2023 |
et al., Elsevier BV, doi:10.2139/ssrn.4488145 | Clinical Prognosis and Risk Factors of Death for Covid-19 Patients Complicated with Coronary Heart Disease/Diabetes/Hypertension-A Retrospective, Real-World Study |
| 2% lower mortality (p=0.04). Retrospective 1,281 COVID-19 patients with comorbidities in China, showing 2% lower mortality with paxlovid. | ||
Jun 17 2023 |
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.06.016 | Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission |
| 14% lower severe cases (p=0.63) and 35% lower hospitalization (p=0.002). Retrospective 3,959 paxlovid patients and 139,379 untreated controls, showing lower hospitalization with treatment. Contraindicted patients were excluded. | ||
Jun 16 2023 |
et al., Research Square, doi:10.21203/rs.3.rs-3003449/v1 | The association of mortality with vaccination and underlying disease among COVID-19 patients in long term care hospitals at Daegu and Gyeonsangbuk-do in Korea |
| 5% lower mortality (p=0.86). Retrospective 2,507 COVID-19 patients at 18 long term care hospitals with COVID-19 outbreaks in Korea, showing no significant difference in mortality with paxlovid treatment. Note that this study is less affected by the typical confoundin.. | ||
May 14 2023 |
et al., medRxiv, doi:10.1101/2023.05.10.23289325 | Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China |
| 29% lower mortality (p=0.16) and 28% worse recovery (p<0.0001). Retrospective 1,082 hospitalized COVID-19 patients in China, showing lower mortality and worse quality of life with paxlovid. | ||
May 10 2023 |
et al., Elsevier BV, doi:10.2139/ssrn.4444431 | Real-Life Comparison of Mortality in Non-Hospitalised Patients with SARS-CoV-2 Infection at Risk for Clinical Progression Treated with Molnupiravir or Nirmatrevir Plus Ritonavir During the Omicron Era in Italy: A Nationwide, Observational Study |
| 32% lower mortality (p=0.0001). Prospective study of 17,977 outpatients treated with molnupiravir and 11,576 treated with paxlovid, showing significant mortality with both treatments, and lower mortality with paxlovid. | ||
Apr 30 2023 |
et al., Annals of Internal Medicine, doi:10.7326/M22-3057 | Effectiveness of Molnupiravir and Nirmatrelvir–Ritonavir in Hospitalized Patients With COVID-19 |
| 23% lower mortality (p=0.001), 3% higher ventilation (p=0.89), and 8% higher ICU admission (p=0.82). Target trial emulation retrospective with 7,119 patients in Hong Kong, showing lower mortality with paxlovid, but no significant difference for ventilation and ICU admission. See also [acpjournals.org], [acpjournals.org]. | ||
Apr 25 2023 |
et al., Frontiers in Pediatrics, doi:10.3389/fped.2023.1160929 | Clinical efficacy analysis of paxlovid in children with hematological diseases infected with the omicron SARS-CoV-2 new variant |
| 51% worse recovery (p=0.008) and 58% faster viral clearance (p=0.03). Retrospective 20 pediatric hematological disease patients in China, showing faster viral clearance with paxlovid, but slower resolution of fever. | ||
Apr 6 2023 |
et al., medRxiv, doi:10.1101/2023.04.05.23288196 | Incidence of Symptoms Associated with Post-Acute Sequelae of SARS-CoV-2 infection in Non-Hospitalized Vaccinated Patients Receiving Nirmatrelvir-Ritonavir |
| 20% lower PASC (p=0.01). TriNetX retrospective 1,004 paxlovid patients and matched controls, showing lower risk of PASC with treatment. | ||
Mar 23 2023 |
et al., Clinical Therapeutics, doi:10.1016/j.clinthera.2023.03.012 | Prevalence of Potential Drug-Drug Interactions with Ritonavir-Containing COVID-19 therapy in the United States: An Analysis of the National Health and Nutrition Examination Survey |
| Analysis of 15,685 adults in the USA estimating that 29.3% of the population risk a major or contraindicated drug interaction with paxlovid, which may increase significantly for patients over 60 or with several comorbidities that also inc.. | ||
Mar 22 2023 |
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2023.1147980 | Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge |
| 31% lower mortality (p=0.5), 44% lower progression (p=0.04), 14% lower ICU admission (p=0.61), and 9% shorter hospitalization (p=0.03). Retrospective 195 patients with impaired kidney function in China, showing lower combined mortality/ICU/cardiovascular events, and improved viral clearance with paxlovid. | ||
Mar 15 2023 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00118-4 | Effectiveness of nirmatrelvir–ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system |
| 84% lower combined mortality/ICU admission (p=0.03) and 54% lower combined mortality/hospitalization (p=0.03). Retrospective 7,274 outpatients in the USA treated with paxlovid and matched controls, showing lower combined hospitalization/death with treatment. With a small percentage of eligible patients receiving treatment, confounding by indicatio.. | ||
Mar 13 2023 |
et al., Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2221857120 | Computational prediction of interactions between Paxlovid and prescription drugs |
| In Silico analysis of drug–drug interactions for paxlovid. From 2,248 prescription drugs, 1,628 were predicted to have 2,445 interactions with nirmatrelvir and/or ritonavir (673 for nirmatrelvir and 1,403 ritonavir). For 873 drugs, author.. | ||
Feb 22 2023 |
et al., Journal of Infection, doi:10.1016/j.jinf.2023.02.029 | Molnupiravir and nirmatrelvir-ritonavir reduce mortality risk during post-acute COVID-19 phase |
| 28% lower mortality (p<0.0001), 24% lower hospitalization (p<0.0001), and 18% lower progression (p<0.0001). Retrospective 30,040 hospitalized patients in Hong Kong, showing lower mortality with paxlovid treatment. Patients with contraindications to paxlovid were not excluded. | ||
Feb 10 2023 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkad027 | Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo |
| In Vitro and animal study comparing nirmatrelvir and ensitrelvir, showing similar efficacy in vitro, and equal or better efficacy of ensitrelvir in vivo (with similar unbound-drug plasma concentrations). | ||
Feb 9 2023 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00011-7 | Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study |
| Retrospective 28,167 patients in the USA demonstrating confounding. The large difference shown in Figure 1 at day 0 indicates that the groups are not comparable (32 control hospitalizations versus 0 for paxlovid at day 0) and suggests imm.. | ||
Feb 5 2023 |
et al., The Lancent Regional Health, doi:10.1016/j.lanwpc.2023.100694 | Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study |
| 38% lower mortality (p=0.57), 67% higher ventilation (p=0.44), 10% longer ICU admission (p=0.8), and 27% higher progression (p=0.58). RCT 264 patients in China, showing no significant difference in outcomes with paxlovid. | ||
Jan 31 2023 |
et al., American Journal of Transplantation, doi:10.1016/j.ajt.2022.12.004 | Paxlovid associated with decreased hospitalization rate among adults with COVID-19 — United States, April–September 2022 |
| 51% lower hospitalization (p<0.0001). Retrospective 699,848 outpatients with COVID-19 showing lower hospitalization with paxlovid. | ||
Jan 25 2023 |
et al., Journal of Infection, doi:10.1016/j.jinf.2023.02.012 | Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study |
| 41% lower combined mortality/hospitalization (p=0.04). Retrospective high risk outpatients in the UK, showing lower hospitalization/death with paxlovid treatment. Residual confounding is likely with adjustments having no detail on specific comorbidities. Patients with contraindications for pa.. | ||
Jan 22 2023 |
et al., medRxiv, doi:10.1101/2023.01.20.23284849 | Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform |
| 4% higher combined mortality/hospitalization (p=0.91). OpenSAFELY retrospective 7,683 outpatients in the UK, showing no significant difference in hospitalization/death between paxlovid and sotrovimab. | ||
Jan 11 2023 |
et al., Science Translational Medicine, doi:10.1126/scitranslmed.abq7360 | SARS-CoV-2 3CLpro mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376 |
| In Vitro and In Silico study showing selection of resistant mutations with nirmatrelvir use. Several mutations were identified that confer resistance to 3CLpro inhibitors nirmatrelvir, ensitrelvir, and GC376. Authors note that most of the.. | ||
Jan 10 2023 |
et al., mBio, doi:10.1128/mbio.02815-22 | The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir |
| In Vitro study showing selection of nirmatrelvir-resistant mutations with a protease inhibitor. | ||
Dec 13 2022 |
et al., Annals of Internal Medicine, doi:10.7326/M22-2141 (date from preprint) | Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System |
| 71% lower mortality (p=0.006), 44% lower combined mortality/hospitalization (p=0.0001), and 40% lower hospitalization (p=0.001). IPW retrospective 44,551 outpatients age 50+ in the USA, showing lower mortality and hospitalization with paxlovid treatment. | ||
Dec 6 2022 |
et al., medRxiv, doi:10.1101/2022.12.05.22283134 | Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes |
| 58% lower mortality (p=0.0004), 56% lower ICU admission (p=0.03), and 7% lower hospitalization (p=0.53). Retrospective 112,380 high-risk patients in the USA, showing lower mortality with paxlovid treatment. The title and headers of Table S14 are conflicting but the data appears to match the title. | ||
Nov 15 2022 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140 | Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease |
| Retrospective 62,525 hospitalized COVID-19 patients in France, showing that over 50% of patients that died had a contraindication for the use of Paxlovid. Retrospective studies that do not exclude contraindicated patients may significantl.. | ||
Nov 15 2022 |
et al., medRxiv, doi:10.1101/2022.11.14.22282195 | The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants |
| 1% improved recovery (p=0.9), 3% improved viral clearance (p=0.73), and 171% worse results (p=0.06). Prospective study of 170 COVID-19 patients in the USA, showing no significant difference in symptomatic and viral recovery times, and higher risk of symptomatic rebound, without statistical significance. There were more elderly patients i.. | ||
Nov 5 2022 |
et al., medRxiv, doi:10.1101/2022.11.03.22281881 | Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada |
| 50% lower mortality (p<0.0001) and 43% lower combined mortality/hospitalization (p<0.0001). Retrospective 177,545 patients in Canada, 8,876 treated with paxlovid, showing lower mortality and hospitalization with treatment, and declining efficacy over the two time periods analyzed. | ||
Oct 8 2022 |
et al., The Lancet, doi:10.1016/S0140-6736(22)01586-0 | Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study |
| 66% lower mortality (p<0.0001), 38% lower ventilation (p=0.36), 58% higher ICU admission (p=0.08), and 24% lower hospitalization (p<0.0001). PSM retrospective 1,074,856 outpatients in Hong Kong, showing lower mortality and hospitalization with paxlovid. | ||
Sep 14 2022 |
et al., medRxiv, doi:10.1101/2022.09.13.22279908 | Real-World Effectiveness of Nirmatrelvir/Ritonavir in Preventing Hospitalization Among Patients With COVID-19 at High Risk for Severe Disease in the United States: A Nationwide Population-Based Cohort Study |
| 73% lower mortality (p<0.0001) and 84% lower hospitalization (p<0.0001). Pfizer retrospective 2,811 high risk COVID-19 patients treated with paxlovid in the US, and 10,849 matched controls, showing lower risk of mortality and hospitalization with treatment. | ||
Sep 6 2022 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.980002 | The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial |
| 15% faster recovery (p=0.26) and 16% faster viral clearance (p=0.009). Retrospective 106 paxlovid and 36 control patients in China, showing faster viral clearance with treatment. | ||
Aug 28 2022 |
et al., Vaccines, doi:10.3390/vaccines10091409 | Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China |
| 20% faster viral clearance (p=0.0006). Retrospective 226 patients in China, showing faster viral clearance with paxlovid. Age range and severity differed between groups. | ||
Aug 20 2022 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac673 | Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with Covid-19 |
| 95% lower mortality (p=0.002), 39% lower progression (p=0.0001), and 56% lower hospitalization (p=0.02). TriNetX retrospective 1,131 vaccinated COVID-19 patients treated with paxlovid and matched controls, showing lower mortality and hospitalization with treatment. | ||
Aug 9 2022 |
et al., Research Square, doi:10.21203/rs.3.rs-1915291/v1 | H172Y mutation perturbs the S1 pocket and nirmatrelvir binding of SARS-CoV-2 main protease through a nonnative hydrogen bond |
| In Silico and In Vitro study of the H172Y mutation which significantly reduces paxlovid's inhibitory activity. Monotherapy with paxlovid and selective pressure may favor resistance mutations. | ||
Aug 4 2022 |
et al., Emerging Microbes & Infections, doi:10.1080/22221751.2022.2109517 | Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai Omicron wave |
| 12% improved viral clearance (p=0.02). Retrospective 1,377 patients in China, showing significantly faster viral clearance with Paxlovid. Authors analyze progression to severe/critical disease, but do not provide results for Paxlovid. | ||
Jul 23 2022 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac600 | Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19 |
| 79% improved viral clearance (p<0.0001). Retrospective 258 paxlovid patients and 224 patients before paxlovid was available in China, showing significantly faster viral clearance with treatment. Adjusted results are only provided for subgroups (≤5, >5 days from onset). Patients .. | ||
Jul 6 2022 |
et al., medRxiv, doi:10.1101/2022.07.03.22277169 | Clinical advancement of patients infected with SARS-CoV-2 Omicron variant in Shanghai, China |
| 20% faster viral clearance (p=0.0006). Retrospective 17 paxlovid and 114 lianhuaqingwen patients in China, showing faster viral clearance with paxlovid. | ||
Jun 14 2022 |
, NCT05011513 | Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR) |
| 52% lower hospitalization (p=0.2), 4% lower progression (p=0.21), 8% faster recovery (p=0.16), and 4% improved viral clearance (p=0.04). Results for the terminated and unpublished (until April 2024) EPIC-SR trial. | ||
Jun 2 2022 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac443 | Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients |
| 46% lower severe cases (p=0.0002). Retrospective 180,351 patients in Israel, 4,737 treated with paxlovid, showing significantly lower combined severe COVID-19 / mortality with treatment. | ||
Jun 1 2022 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2204919 (date from preprint) | Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge |
| 56% lower mortality (p=0.37) and 57% lower hospitalization (p=0.1). Retrospective 109,254 patients in Israel, 3,902 treated with nirmatrelvir, showing lower mortality and hospitalization with treatment for the subgroup of patients >65. Authors only provide subgroup results. | ||
May 24 2022 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac687 (date from preprint) | Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients |
| 19% lower combined mortality/ICU admission (p=0.45) and 21% lower hospitalization (p=0.01). Propensity score weighted retrospective of 93,883 outpatients in Hong Kong, 5,808 treated with molnupiravir and 4,921 treated with paxlovid, showing higher hospitalization and higher combined mortality/mechanical ventilation/ICU admission.. | ||
May 20 2022 |
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(22)00507-2 (date from preprint) | Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study |
| 66% lower mortality (p<0.0001), 3% lower ventilation (p=0.96), 43% lower progression (p<0.0001), and 4% shorter hospitalization (p=0.32). PSM retrospective 40,776 patients in Hong Kong, showing lower mortality and lower combined mortality, ventilation, ICU, and oxygen therapy with paxlovid treatment. | ||
Apr 29 2022 |
, Press Release | Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use |
| 25% lower severe cases (p=0.42), 15% lower progression (p=0.2), and 36% fewer symptomatic cases (p=0.12). PEP RCT showing lower risk of cases with treatment, without statistical significance. Results from [classic.clinicaltrials.gov]. | ||
Apr 4 2022 |
et al., Nature, doi:10.1038/d41586-022-00919-5 | African clinical trial denied access to key COVID drug Paxlovid |
| News reporting that Pfizer denied access to Paxlovid for an independent trial. The press release indicates that Pfizer has denied access for multiple groups [ANTICOV]. Also see [twitter.com]. | ||
Mar 15 2022 |
, Press Release | Pfizer blocking research to generate evidence on optimal use of novel antiviral for COVID-19 patients in low- and middle-income countries |
| ANTICOV reports that Pfizer has denied access to paxlovid for studies proposed by multiple groups. Also see [Ledford]. | ||
Feb 16 2022 |
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2118542 | Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19 |
| 96% lower mortality (p=0.0005), 88% lower hospitalization (p<0.0001), 11% higher progression (p=0.45), and 21% faster recovery (p=0.0003). EPIC-HR RCT, 1,039 higher risk patients treated with paxlovid (PF-07321332 + ritonavir) and 1,046 control patients, showing significantly lower mortality and hospitalization with treatment. | ||
Dec 14 2021 |
, Press Release | Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk or hospitalization or death |
| 70% lower hospitalization (p=0.05). EPIC-SR trial interim results, 428 patients treated with paxlovid (PF-07321332 + ritonavir) and 426 control patients, showing lower hospitalization with treatment. NCT05011513. | ||
Nov 30 2021 |
et al., bioRxiv, doi:10.1101/2021.11.28.4702264 | Main protease mutants of SARS-CoV-2 variants remain susceptible to PF-07321332 |
| In Vitro study showing that PF-07321332 maintains efficacy against variants C.37 lambda, B.1.1.318, B.1.2, B.1.351 beta, and P.2 zeta. | ||
Nov 5 2021 |
et al., bioRxiv, doi:10.1101/2021.11.04.467077 | The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern |
| In Vitro and hamster study showing paxlovid component PF-07321332 effective against four variants of concern, inibits replication of the alpha variant in primary human airway epithelial cells, protected Syrian hamsters against intranasal .. | ||
Jul 30 2021 |
et al., International Journal of Clinical Pharmacy, doi:10.1007/s11096-021-01311-5 | Drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19: a disproportionality analysis of U.S. food and drug administration adverse event reporting system (FAERS) data |
| Disproportionality analysis showing higher risk of liver injury with lopinavir/ritonavir for COVID-19 patients. Paxlovid combines nirmatrelvir and ritonavir. | ||
Aug 22 2002 |
et al., Arteriosclerosis, Thrombosis, and Vascular Biology, doi:10.1161/01.atv.0000034707.40046.02 | HIV Protease Inhibitor Ritonavir Induces Cytotoxicity of Human Endothelial Cells |
| In Vitro study showing that ritonavir (part of paxlovid) can cause endothelial mitochondrial DNA damage and cell death at concentrations near clinical plasma levels. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. FLCCC and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.