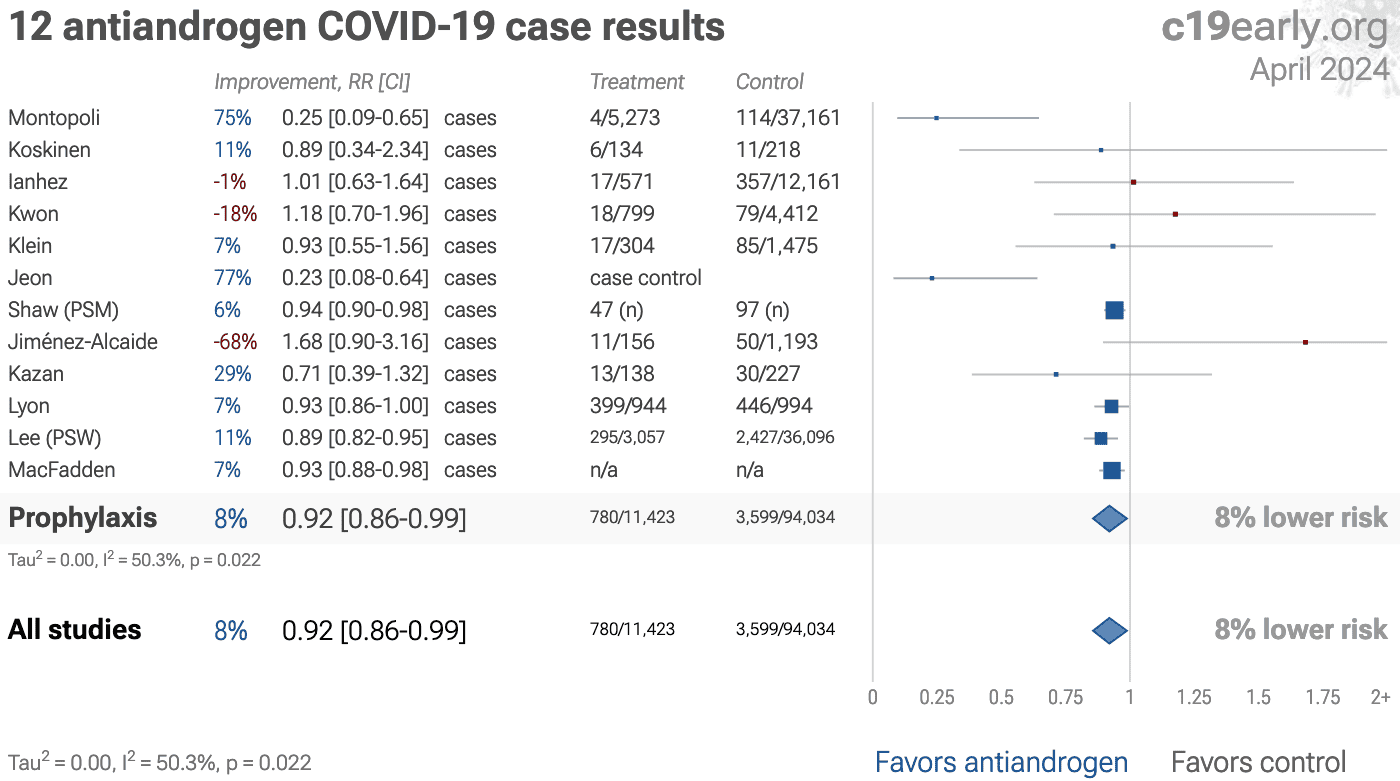
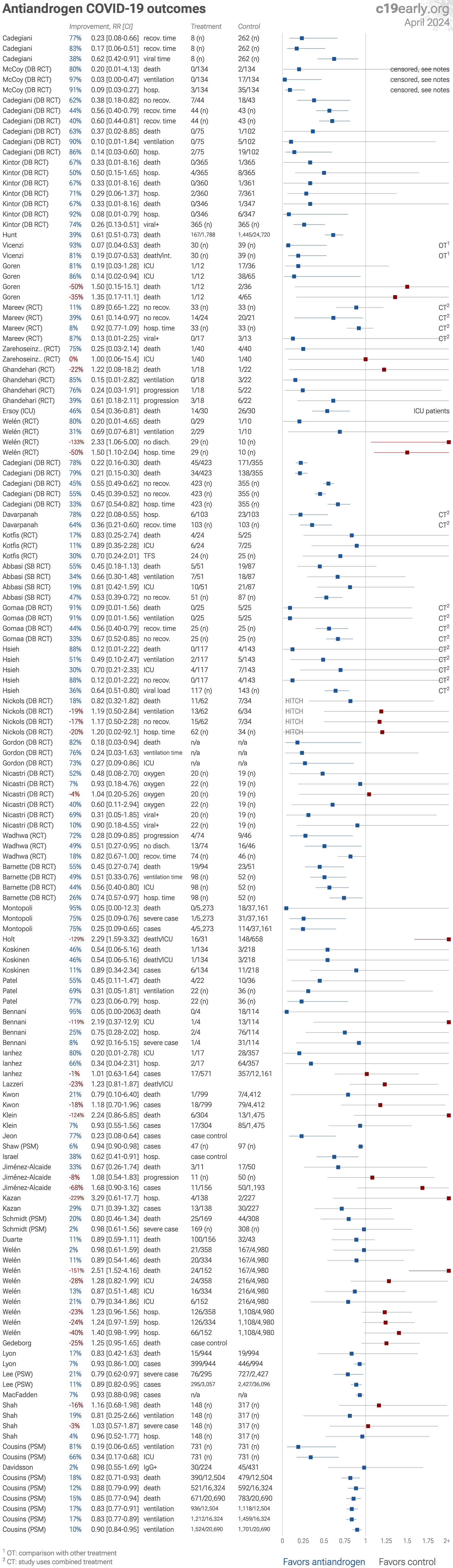
This analysis combines the results of several different antiandrogens. Results for individual treatments may vary.
Recent:Mohta.
Submit updates/corrections .
Apr 28 |
Antiandrogens for COVID-19: real-time meta analysis of 49 studies | |
| Statistically significant lower risk is seen for mortality, ventilation, ICU admission, hospitalization, recovery, cases, and viral clearance. 29 studies from 23 independent teams in 12 countries show statistically significant improvement.. | ||
Feb 13 |
et al., Indian Dermatology Online Journal, doi:10.4103/idoj.idoj_222_23 | The Association between COVID-19 Infection and Gabrin Sign: A Case-Control Study |
| Retrospective case-control study of 540 COVID-19 patients hospitalized in India, showing higher COVID-19 severity and mortality among those with androgenetic alopecia. Cases were further divided into those with more severe alopecia (Gabri.. | ||
Nov 15 2023 |
et al., Journal of Ayurveda and Integrative Medicine, doi:10.1016/j.jaim.2023.100778 | Effectiveness of ayurvedic formulation, NAOQ19 along with standard care in the treatment of mild-moderate COVID-19 patients: A double blind, randomized, placebo-controlled, multicentric trial |
| 13% shorter hospitalization (p=0.05) and 12% faster viral clearance (p=0.02). RCT 150 mild-moderate COVID-19 patients showing faster viral clearance and shorter hospitalization with NAOQ19, a polyherbal formulation containing 13 antiviral/antinflammatory compounds. NAOQ19 ingredients include curcumin, andrographis,.. | ||
Apr 18 2023 |
et al., Journal of Medical Virology, doi:10.1002/jmv.28740 | Antiandrogens for the treatment of COVID‐19 patients: A meta‐analysis of randomized controlled trials |
| 63% lower mortality (p<0.0001). Meta analysis of 14 antiandrogen RCTs for COVID-19, showing significantly lower mortality with treatment. | ||
Mar 2 2023 |
et al., medRxiv, doi:10.1101/2023.02.28.23286515 | Association between spironolactone use and COVID-19 outcomes in population-scale claims data: a retrospective cohort study |
| 18% lower mortality (p=0.004) and 17% lower ventilation (p<0.0001). PSM retrospective 898,303 hospitalized COVID-19 patients in the USA, 16,324 on spironolactone, showing lower mortality and ventilation with spironolactone use. | ||
Feb 7 2023 |
et al., International Journal of Molecular Sciences, doi:10.3390/ijms24043288 | Apalutamide Prevents SARS-CoV-2 Infection in Lung Epithelial Cells and in Human Nasal Epithelial Cells |
| In Vitro study showing that TMPRSS2 expression is regulated by androgens in Calu-3 cells, and treatment with anti-androgen drugs such as apalutamide significantly reduced SARS-CoV-2 entry and infection in both Calu-3 lung cells and primar.. | ||
Jan 19 2023 |
et al., The Prostate, doi:10.1002/pros.24485 | Androgen deprivation therapy in men with prostate cancer is not associated with COVID‐2019 infection |
| 2% lower IgG positivity (p=0.95). Retrospective 655 prostate cancer patients in Sweden, showing no significant difference in seropositivity with ADT. | ||
Aug 4 2022 |
et al., SSRN Electronic Journal, doi:10.2139/ssrn.4181700 | Antiandrogen Agents in COVID-19: A Meta-Analysis of Randomized Trials |
| 60% lower mortality (p=0.0004) and 53% lower progression (p=0.003). Meta analysis of 12 studies, showing significantly lower mortality with antiandrogen treatment. | ||
Jul 6 2022 |
et al., Cell Reports Methods, doi:10.1016/j.crmeth.2023.100503 (date from preprint) | Integrative analysis of functional genomic screening and clinical data identifies a protective role for spironolactone in severe COVID-19 |
| 81% lower ventilation (p=0.006) and 66% lower ICU admission (p=0.002). PSM retrospective 64,349 COVID-19 patients in the USA, showing spironolactone associated with lower ICU admission. Authors also present In Vitro research showing dose-dependent inhibition in a human lung epithelial cell line. | ||
Jul 6 2022 |
et al., NEJM Evidence, doi:10.1056/EVIDoa2200145 | Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis |
| 55% lower mortality (p=0.002), 49% shorter ventilation (p=0.001), 44% shorter ICU admission (p=0.001), and 26% shorter hospitalization (p=0.03). RCT with 98 hospitalized moderate/severe patients treated with sabizabulin and 52 control patients, showing lower mortality with treatment. Sabizabulin 9mg for up to 21 days. For more discussion see [twitter.com, twitter.com, twitter.com]. | ||
Jul 2 2022 |
et al., medRxiv, doi:10.1101/2022.07.01.22277163 | Phase 2 randomised placebo-controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID-19 hospitalised patients in Delhi |
| 72% lower progression (p=0.03), 49% higher hospital discharge (p=0.05), and 18% faster recovery (p=0.06). RCT 120 hospitalized patients in India, 74 treated with spironolactone and dexamethasone, and 46 with dexamethasone, showing lower progression with treatment. Spironolactone 50mg once daily day 1, 25mg once daily until day 21. | ||
Jun 30 2022 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101450 | A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19 |
| 52% lower need for oxygen therapy (p=0.43) and 69% improved viral clearance (p=0.22). RCT 68 patients in Italy showing improved viral clearance with raloxifene. | ||
Jun 29 2022 |
et al., Journal of General Internal Medicine, doi:10.1007/s11606-022-07701-3 | Medications Associated with Lower Mortality in a SARS-CoV-2 Positive Cohort of 26,508 Veterans |
| 39% lower mortality (p<0.0001). Retrospective 26,508 consecutive COVID+ veterans in the USA, showing lower mortality with multiple treatments including anti-androgens. Treatment was defined as drugs administered ≥50% of the time within 2 weeks post-COVID+, and may be a .. | ||
May 12 2022 |
et al., JNCI Cancer Spectrum, doi:10.1093/jncics/pkac035 | The Impact of Androgen Deprivation Therapy on COVID-19 Illness in Men With Prostate Cancer |
| 16% higher mortality (p=0.59), 19% lower ventilation (p=0.73), 3% higher severe cases (p=0.91), and 4% lower hospitalization (p=0.9). Retrospective 465 prostate cancer patients, showing no significant difference in COVID-19 outcomes with ADT. | ||
May 10 2022 |
et al., European Journal of Endocrinology, doi:10.1530/EJE-22-0104 | Higher premorbid serum testosterone predicts COVID-19-related mortality risk in men. |
| Prospective UK Biobank study showing higher premorbid testosterone levels associated with higher COVID-19 mortality. | ||
May 5 2022 |
et al., Physical Chemistry Research, doi:10.22036/pcr.2022.324549.2016 | In-Silico Molecular Docking, Validation, Drug-Likeness, and ADMET Studies of Antiandrogens to Use in the Fight against SARS-CoV-2 |
| In Silico study of several antiandrogens identifying strong candidates for inhibition of SARS-CoV-2. Apalutamide and bicalutamide showed the best binding affinity against TMPRSS2. | ||
Apr 25 2022 |
, M., 32nd European Congress of Clinical Microbiology & Infectious Diseases | Phase 2 study of oral sabizabulin for the treatment of SARS-CoV-2 in hospitalized patients at high risk for ARDS |
| 82% lower mortality (p=0.04), 76% shorter ventilation (p=0.14), and 73% shorter ICU admission (p=0.03). Phase 2 RCT of sabizabulin showing lower mortality with treatment. For more discussion see [twitter.com]. | ||
Apr 19 2022 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2022.7852 | Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19 |
| 18% lower mortality (p=0.66), 19% higher ventilation (p=0.7), 17% worse results (p=0.7), and 20% longer hospitalization (p=0.94). Early terminated RCT with 62 very late stage (79% on oxygen) degarelix patients and 34 placebo patients, showing no significant differences with treatment. For discussion of many issues with this study see [twitter.com]. | ||
Apr 5 2022 |
, Press Release | Kintor Pharma's Proxalutamide Demonstrated Reduction in Hospitalization/Mortality for Patients with Mild to Moderate COVID-19 in Phase III MRCT Study |
| 50% lower hospitalization (p=0.38) and 74% improved viral clearance (p=0.0001). RCT 733 outpatients, 99% in the USA, showing lower hospitalization/death, and significantly reduced viral load with proxalutamide treatment. The viral clearance result is from . | ||
Mar 31 2022 |
et al., Journal of Infection, doi:10.1016/j.jinf.2022.03.020 | Protective trend of anti-androgen therapy during the COVID-19 pandemic: A meta-analysis |
| Meta analysis of 15 antiandrogen studies showing a protective trend although there has been conflicting results to date. | ||
Mar 29 2022 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac156 | Screening Large Population Health Databases for Potential COVID-19 Therapeutics: A Pharmacopeia-Wide Association Study (PWAS) of Commonly Prescribed Medications |
| 7% fewer cases (p=0.008). Retrospective 26,121 cases and 2,369,020 controls ≥65yo in Canada, showing lower cases with chronic use of spironolactone. | ||
Mar 14 2022 |
et al., Frontiers in Nutrition, doi:10.3389/fnut.2022.832321 | Efficacy and Safety of Complementary Therapy With Jing Si Herbal Tea in Patients With Mild-To-Moderate COVID-19: A Prospective Cohort Study |
| 88% lower mortality (p=0.13), 30% lower ICU admission (p=0.76), 88% improved recovery (p=0.13), and 36% improved viral clearance (p=0.0002). Prospective study of 260 hospitalized patients in Taiwan, 117 treated with herbal formula Jing Si Herbal Tea which includes antiandrogen glycyrrhiza glabra, showing improved recovery with treatment, with statistical significance for SpO2,.. | ||
Mar 8 2022 |
et al., medRxiv, doi:10.1101/2022.03.05.22271959 | 13 cis retinoic acid improved the outcomes of COVID-19 patients. A randomized clinical trial |
| 86% lower mortality (p=0.23), 67% lower ICU admission (p=0.24), 35% faster recovery (p<0.0001), and 44% faster viral clearance (p<0.0001). RCT with 20 13-cis-retinoic acid patients and 20 control patients, showing faster recovery and viral clearance with treatment. Aerosolized 13-cis-retinoic acid with increasing dose from 0.2 mg/kg/day to 4 mg/kg/day for 14 days, plus oral.. | ||
Mar 7 2022 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.774773 | A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity |
| 21% lower severe cases (p=0.03) and 11% fewer cases (p=0.001). Retrospective 3,057 androgen deprivation therapy patients in the USA, and 36,096 control patients with cancer, showing lower risk of cases and severity with ADT. | ||
Mar 1 2022 |
et al., Inflammopharmacology, doi:10.1007/s10787-022-00939-7 | Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial |
| 91% lower mortality (p=0.05), 91% lower ventilation (p=0.05), and 44% faster recovery (p=0.001). RCT with 50 hospitalized COVID+ patients in Egypt, 25 treated with glycyrrhizin and boswellic acid, showing improved recovery with treatment. Glycyrrhizin 60mg and boswellic acid 200mg bid for 2 weeks. NCT04487964. | ||
Feb 24 2022 |
et al., Journal of Clinical Medicine Research, doi:10.14740/jocmr4658 | “MATH+” Multi-Modal Hospital Treatment Protocol for COVID-19 Infection: Clinical and Scientific Rationale |
| Review of the data supporting the MATH+ hospital treatment protocol for COVID-19. | ||
Feb 7 2022 |
et al., Journal of the Endocrine Society, doi:10.1210/jendso/bvac017 | A Randomized Trial of Sitagliptin and Spironolactone With Combination Therapy in Hospitalized Adults With COVID-19 |
| 55% lower mortality (p=0.1), 34% lower ventilation (p=0.36), 19% lower ICU admission (p=0.67), and 47% improved recovery (p<0.0001). RCT including 51 spironolactone patients and 87 control patients in Iran, showing improved recovery with spironolactone, sitagliptin, and the combination of both. | ||
Feb 5 2022 |
et al., Pharmaceuticals, doi:10.3390/ph15020200 | Mineralocorticoid Receptor Antagonist (Potassium Canrenoate) Does Not Influence Outcome in the Treatment of COVID-19-Associated Pneumonia and Fibrosis—A Randomized Placebo Controlled Clinical Trial |
| 17% lower mortality (p=1), 11% lower ICU admission (p=1), and 30% improved recovery (p=0.51). RCT with 24 patients treated with potassium canrenoate and 25 placebo patients in Poland, showing no significant differences. | ||
Jan 31 2022 |
et al., Journal of Urology, doi:10.1097/JU.0000000000002180 | 5α-Reductase Inhibitors Are Associated with Reduced Risk of SARS-CoV-2 Infection: A Matched-Pair, Registry-Based Analysis |
| 17% lower mortality (p=0.61) and 7% fewer cases (p=0.04). Retrospective 944 5ARI users in the USA and 944 matched controls, showing lower risk of COVID-19 cases with treatment. | ||
Jan 21 2022 |
et al., medRxiv, doi:10.1101/2022.01.21.22269322 | Combination of Spironolactone and Sitagliptin Improves Clinical Outcomes of Outpatients with COVID-19: An Observational Study |
| 78% lower hospitalization (p=0.0008) and 64% faster recovery (p=0.0001). Prospective study of 206 outpatients in Iran, 103 treated with spironolactone and sitagliptin, showing lower hospitalization and faster recovery with treatment. spironolactone 100mg and sitagliptin 100mg daily. | ||
Dec 27 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-1165680/v1 | An integrative approach to clinical recovery for COVID-19 patients using an Ayurvedic formulation: A multicentric double-blind randomized control trial |
| 89% improved recovery (p=0.05) and 24% improved viral clearance (p=0.47). Small RCT with 39 patients treated with NOQ19 and 37 placebo patients, showing improved recovery, without statistical significance. NOQ19 has multiple ingredients including curcumin, andrographis, and antiandrogen glycyrrhiza glabra. | ||
Dec 25 2021 |
et al., Cureus, doi:10.7759/cureus.20691 | Final Results of a Randomized, Placebo-Controlled, Two-Arm, Parallel Clinical Trial of Proxalutamide for Hospitalized COVID-19 Patients: A Multiregional, Joint Analysis of the Proxa-Rescue AndroCoV Trial |
| 78% lower mortality (p<0.0001), 45% improved recovery (p<0.0001), and 33% shorter hospitalization (p=0.0001). RCT 778 hospitalized patients in Brazil, 423 treated with proxalutamide, showing significantly lower mortality and improved recovery with treatment. NCT04728802 and NCT05126628. Authors note that cases in this trial were predominantly the.. | ||
Dec 23 2021 |
et al., Scandinavian Journal of Urology, doi:10.1080/21681805.2021.2019304 | Androgen deprivation therapy, comorbidity, cancer stage and mortality from COVID-19 in men with prostate cancer |
| 25% higher mortality (p=0.11). Case control study with 474 patients that died of COVID-19 in Sweden, showing higher risk with ADT, without statistical significance. | ||
Dec 14 2021 |
et al., European Urology, doi:10.1016/j.eururo.2021.12.013 | A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data |
| 80% lower mortality (p=0.26), 133% lower hospital discharge (p=0.03), and 50% longer hospitalization (p=0.01). Very small late stage RCT with 10 control patients and 29 enzalutamide patients, showing mixed results. Discharge and hospitalization time favored the control group, while viral load reduction was better with treatment on days 4&6 (day 4 .. | ||
Dec 14 2021 |
et al., European Urology, doi:10.1016/j.eururo.2021.12.013 | A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data |
| 2% lower mortality (p=0.94), 28% higher ICU admission (p=0.28), and 23% higher hospitalization (p=0.09). Retrospective 7,894 COVID+ prostate cancer patients, analyzing patients on antiandrogen treatment, ADT, and ADT + abiraterone acetate or enzalutamide, showing mixed results and higher mortality for ADT + abiraterone acetate or enzalutamid.. | ||
Nov 25 2021 |
et al., Infectious Agents and Cancer, doi:10.1186/s13027-021-00406-y | Impact of androgen deprivation therapy on mortality of prostate cancer patients with COVID-19: a propensity score-based analysis |
| 11% lower mortality (p=0.37). Retrospective 199 prostate cancer patients hospitalized with COVID-19 in Brazil, showing no significant difference in mortality with active ADT. | ||
Nov 12 2021 |
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.34330 | Association Between Androgen Deprivation Therapy and Mortality Among Patients With Prostate Cancer and COVID-19 |
| 20% lower mortality (p=0.41) and 2% lower severe cases (p=0.94). Retrospective 1,106 prostate cancer patients, showing no significant differences in COVID-19 outcomes with ADT. | ||
Nov 1 2021 |
et al., Türk Üroloji Dergisi/Turkish Journal of Urology, doi:10.5152/tud.2021.21278 | The clinical impact of androgen deprivation therapy on SARS-CoV-2 infection rates and disease severity |
| 229% higher hospitalization (p=0.2) and 29% fewer cases (p=0.32). Retrospective 365 prostate cancer patients in Turkey, 138 treated with ADT, showing no significant differences with treatment. | ||
Oct 13 2021 |
et al., Aydin Sağlik Dergi̇si̇, doi:10.17932/IAU.ASD.2015.007/asd_v07i3002 | Assessment Of The Efficacy Of Spironolactone For COVID-19 ARDS Patients |
| 46% lower mortality (p=0.002). Retrospective 30 COVID-19 ARDS ICU patients and 30 control patients, showing lower mortality with treatment. | ||
Sep 13 2021 |
et al., The Prostate, doi:10.1002/pros.24232 | Influence of androgen deprivation therapy on the severity of COVID-19 in prostate cancer patients |
| 33% lower mortality (p=0.41), 8% higher progression (p=0.77), and 68% more cases (p=0.15). Retrospective 1,349 prostate cancer patients in Spain, 156 on ADT, showing no significant differences in COVID-19 outcomes with treatment. | ||
Jul 31 2021 |
et al., Chest, doi:10.1016/j.chest.2021.02.024 | Progesterone in Addition to Standard of Care vs Standard of Care Alone in the Treatment of Men Hospitalized With Moderate to Severe COVID-19 |
| 85% lower ventilation (p=0.24), 76% lower progression (p=0.2), and 100% improved recovery (p=0.02). RCT 42 hospitalized patients in the USA, showing improved recovery and lower progression with progesterone treatment. | ||
Jul 27 2021 |
et al., Epidemiology and Global Health Microbiology and Infectious Disease, doi:10.7554/eLife.68165 | Identification of drugs associated with reduced severity of COVID-19: A case-control study in a large population |
| 38% lower hospitalization (p=0.01). Case control study examining medication usage with a healthcare database in Israel, showing lower risk of hospitalization with dutasteride. | ||
Jul 10 2021 |
et al., medRxiv, doi:10.1101/2021.07.06.21260086 | Proxalutamide (GT0918) Reduces the Rate of Hospitalization in mild-to-moderate COVID-19 Female Patients: A Randomized Double-Blinded Placebo-Controlled Two-Arm Parallel Trial |
| 90% lower ventilation (p=0.07) and 86% lower hospitalization (p=0.0008). RCT 177 women in Brazil, 75 treated with proxalutamide, showing significantly lower hospitalization with treatment. | ||
Jul 1 2021 |
et al., Journal of Drugs in Dermatology, doi:10.36849/JDD.5843 | COVID-19 in Individuals Treated With Long-Term Hydroxychloroquine: A Propensity Score-Matched Analysis of Cicatricial Alopecia Patients |
| 6% fewer cases (p=0.006). PSM retrospective 144 alopecia patients in the USA, showing no significant difference in COVID-19 cases with anti-androgen use. The supplemental appendix is not available. | ||
Jul 1 2021 |
et al., Nature Communications, doi:10.1038/s41467-021-24342-y | The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells |
| In Vitro and animal study showing that the antiandrogen enzalutamide reduces TMPRSS2 levels in human lung cells and in mouse lungs. | ||
Jun 5 2021 |
et al., Endocrinology, doi:10.1210/endocr/bqab114 | Do Anti-androgens Have Potential as Therapeutics for COVID-19? |
| Review of research related to the potential benefits of anti-androgrens for COVID-19. | ||
Apr 30 2021 |
et al., Medical Journal of The Islamic Republic of Iran, doi:10.47176/mjiri.35.30 | Finasteride in hospitalized adult males with COVID-19: A risk factor for severity of the disease or an adjunct treatment: A randomized controlled clinical trial |
| 75% lower mortality (p=0.36) and no change in ICU admission (p=1). RCT 80 hospitalized COVID-19 patients in Iran, 40 treated with finasteride, showing no significant differences other than improved oxygen saturation on the 5th day with treatment. There was significantly more patients with diabetes in the.. | ||
Feb 23 2021 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2021.629176 | Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea |
| 77% fewer cases (p=0.005). Retrospective 6,462 liver cirrhosis patients in South Korea, with 67 COVID+ cases, showing significantly lower cases with spironolactone treatment. Death and ICU results per group are not provided. | ||
Feb 22 2021 |
et al., Cureus, doi:10.7759/cureus.13492 | Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial |
| 92% improved viral clearance (p<0.0001) and 77% improved recovery (p<0.0001). RCT 234 mild-moderate COVID-19 patients with 171 treated with proxalutamide, showing significantly faster viral clearance and recovery. Third party analysis suggests potential randomization failure: [steamtraen.blogspot.com]. | ||
Feb 1 2021 |
et al., Journal of Urology, doi:10.1097/JU.0000000000001338 | Androgen Deprivation Therapy in Men with Prostate Cancer Does Not Affect Risk of Infection with SARS-CoV-2 |
| 124% higher mortality (p=0.12) and 7% fewer cases (p=0.8). Retrospective 1,779 prostate cancer patients, showing no significant differences in COVID-19 outcomes with ADT. | ||
Feb 1 2021 |
et al., Cureus, doi:10.7759/cureus.13047 | Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial – Biochemical) |
| 62% improved recovery (p=0.009). RCT 130 outpatients in Brazil, 54 treated with dutasteride, showing faster recovery with treatment. All patients received nitazoxanide. There were no hospitalizations, mechanical ventilation, or deaths. Some percentages for viral clearanc.. | ||
Jan 29 2021 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2021.01.067 | Androgen-deprivation therapy and SARS-CoV-2 in men with prostate cancer: findings from the University of California Health System registry |
| 21% lower mortality (p=1) and 18% more cases (p=0.54). Retrospective 5,211 prostate cancer patients, 799 on ADT, showing no significant differences in COVID-19 outcomes with treatment. | ||
Dec 30 2020 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2021.668698 (date from preprint) | Proxalutamide (GT0918) Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial |
| 97% lower ventilation (p<0.0001) and 91% lower hospitalization (p<0.0001). RCT 268 male patients in Brazil, 134 treated with proxalutamide, showing significantly lower hospitalization and mechanical ventilation. NCT04446429. This paper was censored without details or author response, and the editors have ignored.. | ||
Dec 3 2020 |
et al., Кардиология, doi:10.18087/cardio.2020.11.n1440 | Results of Open-Label non-Randomized Comparative Clinical Trial: “BromhexIne and Spironolactone for CoronаvirUs Infection requiring hospiTalization (BISCUIT) |
| 11% improved recovery (p=0.47), 8% shorter hospitalization (p=0.35), and 87% improved viral clearance (p=0.08). Prospective 103 PCR+ patients in Russia, 33 treated with bromexhine+spironolactone, showing lower PCR+ at day 10 or hospitalization >10 days with treatment. Bromhexine 8mg 4 times daily, spironolactone 25-50 mg/day for 10 days. | ||
Nov 26 2020 |
et al., European Journal of Cancer, doi:10.1016/j.ejca.2020.09.018 | Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer |
| Retrospective study of 34 metastatic castration-resistant prostate cancer (mCRPC) outpatients (median age 75 years) who developed COVID-19, out of 1,433 total mCRPC patients attending participating centers. The study showed 38% COVID-19 m.. | ||
Nov 2 2020 |
et al., Journal of the European Academy of Dermatology and Venereology, doi:10.1111/jdv.17021 | 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia |
| Retrospective 48 androgenetic alopecia patients in Brazil treated with dutasteride, compared with 48 propensity score matched androgenetic alopecia patients not taking any 5ARis, showing a statistically significant reduction in the freque.. | ||
Oct 31 2020 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.06.005 | On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2 |
| Retrospective study of 1,949 metastatic prostate cancer patients on androgen deprivation therapy in Italy. The study found a 1.8% SARS-CoV-2 infection rate and 30.6% mortality among infected patients. Patients were elderly with metastatic.. | ||
Oct 6 2020 |
et al., medRxiv, doi:10.1101/2020.10.05.20206870 | An open-label prospective observational study of antiandrogen and non-antiandrogen early pharmacological approaches in females with mild-to-moderate COVID-19. The Pre-AndroCoV Female Trial |
| 77% faster recovery (p=0.006) and 38% faster viral clearance (p=0.02). Prospective study of 270 female COVID-19 patients in Brazil, 75 with hyperandrogenism, of which 8 were on spironolactone. Results suggest that HA patients may be at increased risk, and that spironolactone use may reduce the risk compared .. | ||
Sep 25 2020 |
et al., Journal of the European Academy of Dermatology and Venereology, doi:10.1111/jdv.16953 | Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men |
| 81% lower ICU admission (p=0.08). Prospective study of 77 men hospitalized with COVID-19, 12 taking antiandrogens (9 dutasteride, 2 finasteride, 1 spironolactone), showing lower ICU admission with treatment (statistically significant with age-matched controls only when exc.. | ||
Sep 21 2020 |
et al., medRxiv, doi:10.1101/2020.04.20.20068056 | Impact of anti-androgen therapies on COVID-19 susceptibility: a case-control study in male population from two COVID-19 regional centers of Lombardy (Italy) |
| 23% higher combined mortality/ICU admission (p=0.33). Retrospective case-control study in Italy with 943 male COVID-19 patients, 45 on chronic 5ARI treatment (finasteride/dutasteride). There was significantly fewer COVID-19 patients >55 on 5ARI treatment compared to age-matched controls (5.5.. | ||
Sep 11 2020 |
et al., Journal of Clinical Medicine, doi:10.3390/jcm9092943 | The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients |
| 93% lower mortality (p<0.0001) and 81% lower combined mortality/intubation (p=0.002). Retrospective 69 consecutive hospitalized COVID-19 patients in Italy, 30 patients receiving canrenone, and 39 treated with vasodilator agents or renin–angiotensin–aldosterone system (RAAS) inhibitors, showing lower mortality with canrenone. | ||
Sep 3 2020 |
et al., Dermatologic Therapy, doi:10.1111/dth.14166 | Androgen sensitivity in |
| 80% lower ICU admission (p=0.26), 66% lower hospitalization (p=0.32), and 1% more cases (p=0.9). Retrospective survey of 41,529 participants, including 571 on antiandrogen therapy, showing no significant association between antiandrogen use and COVID-19 incidence, hospitalization, or ICU admission/mechanical ventilation. | ||
Aug 17 2020 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.08.2095 | Androgen deprivation therapy may constitute a more effective COVID-19 prophylactic than therapeutic strategy |
| 95% lower mortality (p=1), 119% higher ICU admission (p=0.4), 25% lower hospitalization (p=0.6), and 8% lower severe cases (p=1). Retrospective 118 prostate cancer patients, 4 on androgren deprivation therapy, not showing significant differences (as expected with only 4 patients in the treatment group). | ||
Jul 9 2020 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.06.023 | Does androgen deprivation therapy protect against severe complications from COVID-19? |
| 55% lower mortality (p=0.22), 69% lower ventilation (p=0.19), and 77% lower hospitalization (p=0.02). Retrospective 58 prostate cancer patients in the USA, showing lower risk of hospitalization with ADT. | ||
Jun 29 2020 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.06.015 | Androgen deprivation and SARS-CoV-2 in men with prostate cancer |
| 46% lower mortality (p=1), 46% lower combined mortality/ICU admission (p=1), and 11% fewer cases (p=1). Retrospective 352 prostate cancer patients in Finland, showing no significant differences in COVID-19 with ADT. | ||
May 21 2020 |
et al., Journal of the American Academy of Dermatology, doi:10.1016/j.jaad.2020.05.079 | Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign” |
| Analysis of 175 patients hospitalized with severe COVID-19, showing androgenetic alopecia (AGA) in 42% of women and 79% of men. Authors hypothesize that COVID-19 severity is androgen-mediated, and recommend study of antiandrogen treatments. | ||
May 7 2020 |
et al., Journal of Hypertension, doi:10.1097/hjh.0000000000002515 | Influence of inhibitors of the renin–angiotensin system on risk of acute respiratory distress syndrome in Danish hospitalized COVID-19 patients |
| 129% higher combined mortality/ICU admission (p=0.0007). Retrospective 689 hospitalized COVID-19 patients in Denmark, showing higher risk of ICU/death with spironolactone use in unadjusted results subject to confounding by indication. | ||
May 6 2020 |
et al., Annals of Oncology, doi:10.1016/j.annonc.2020.04.479 | Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) |
| 95% lower mortality (p=0.15), 75% lower severe cases (p=0.01), and 75% fewer cases (p=0.004). Retrospective 5,273 prostate cancer patients on androgen-deprivation therapy (ADT), and 37,161 not on ADT, showing lower risk of cases with treatment. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. FLCCC and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.